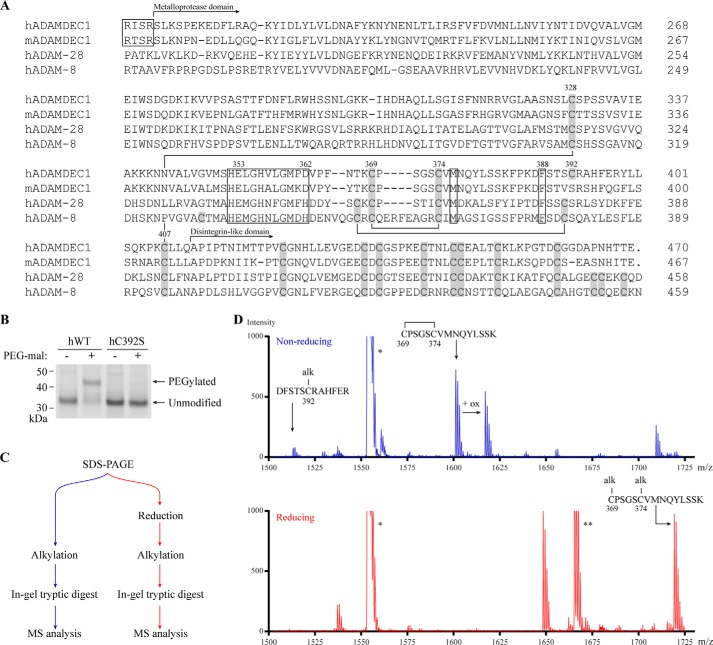
FIGURE 1.
ADAMDEC1 contains a unique Cys pattern. A, multiple sequence alignment comparing the full sequence of mature hADAMDEC1 and mADAMDEC1 with the N-terminal part of mature hADAM-28 and -8. Disulfide bonds in the metalloprotease domain of hADAM-8 are shown by black lines connecting Cys residues (gray boxes) and are based on the crystal structure (23). The metzincin active site, the conserved Met residue in the hallmark Met-turn (Met376), and a conserved structurally important hydrophobic Phe anchor (Phe388) are all marked by black boxes. The identified proprotein convertase-processing sites of human and murine ADAMDEC1 are marked by a separate black box (4, 5). The numbering above selected key residues refers to the hADAMDEC1 full-length sequence. B, thiol-specific PEGylation of hWT and hC392S analyzed by SDS-PAGE. C, experimental setup for tryptic peptide mass fingerprinting using MALDI-TOF mass spectrometry. D, tryptic peptide mass fingerprinting of non-reduced (blue) and reduced (red) human pro-ADAMDEC1 (hADAMDEC1 R56A/R200K/R203A (5)). The mass spectrum of m/z 1500–1730 is shown. Two additional expected ADAMDEC1 tryptic peptides, VVPSASTTFDNFLR (*, residues 279–292) and QTPELTLHEIVCmPK (**, residues 35–48), where Cm denotes a carboxymethylated Cys residue, are also visible in the depicted mass range.