FIGURE 4.
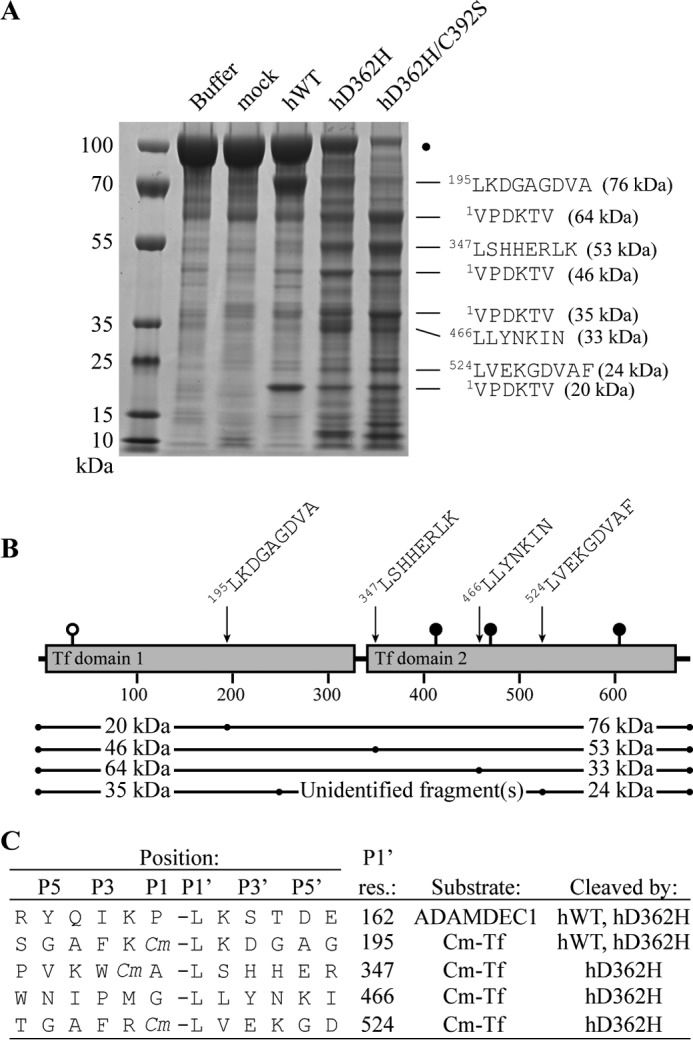
Substrate specificity of hADAMDEC1 variants. A, N-terminal Edman sequencing of the dominant proteolytic bands of Cm-Tf by hADAMDEC1, hD362H, and hD362H/C392S. Mature numbering is used for Cm-Tf. The SDS-polyacrylamide gel is identical to that of Fig. 3B and serves only to illustrate sequenced bands. B, the four identified cleavage sites at residues 195, 347, 466, and 524 giving rise to 76, 53, 33, and 24 kDa product bands, respectively, are mapped on the schematic domain structure of mature transferrin. Suggested fragment pairs making up mature Cm-Tf are shown below. Transferrin is modified by one O-linked and three N-linked glycosylations illustrated by white and black lollipops, respectively. C, sequence alignment of the identified cleavage sites in Cm-Tf and in the ADAMDEC1 prodomain (5). Cm, carboxymethyl-modified Cys residue.
