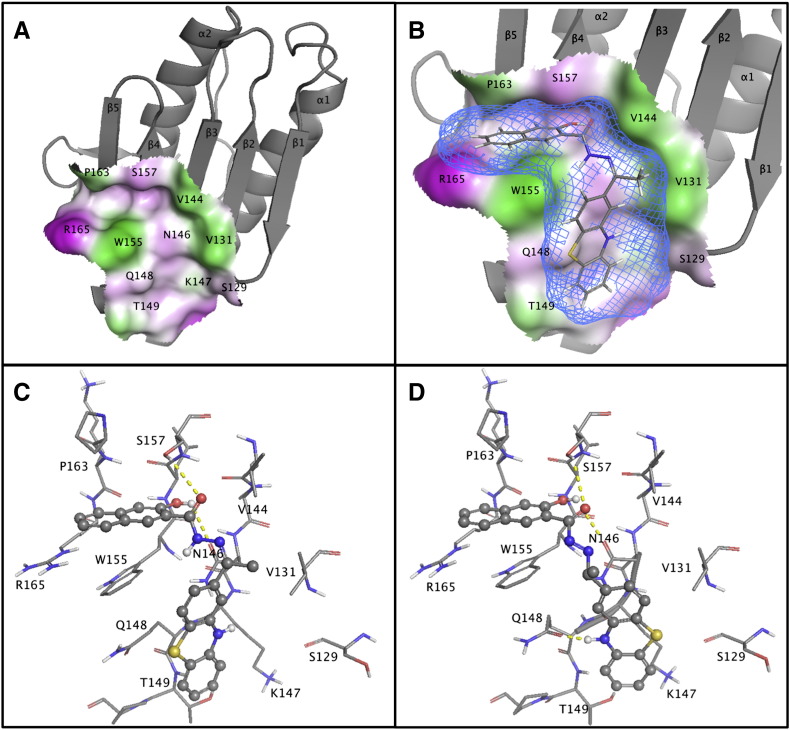
Fig. 1.
Docking model of UCM71 to frataxin.
(A) Crystal structure of frataxin (gray cartoon) with solvent accessible molecular surface around the W155 pocket. Overall structure as grey cartoon, molecular surface colored by lipophilicity (hydrophilic in magenta, lipophilic in green). (B) Putative model of the interaction between W155 pocket and the ligand UCM71 (ball and stick, CPK colors): the solvent accessible ligand surface (light blue mesh) fits perfectly with the naphthyl moiety buried by W155, P163, and S157. The phenothiazine ring recognizes the flat region formed by N146 and K147 (label not shown for clarity) and delimited by side chains of V131, S129, T149 and Q148. (C) Putative selected interactions of UCM71 with frataxin. Hydrogen bonds are formed between the hydroxyl substituent of the naphthyl group and the side chain of N146 and between the carbonyl group of the carbonyl-hydrazone scaffold and the hydroxyl of S157. These interactions induce minor rearrangements in the involved side chains of N146 (flip of terminal amide) and S157. (D) The flexibility of the carbonyl-hydrazone scaffold permits the flip of phenothiazine moiety to point its central amino group toward Q148 possibly inducing the flip of the amide group of its side chain resulting in the formation of a strong hydrogen bond.