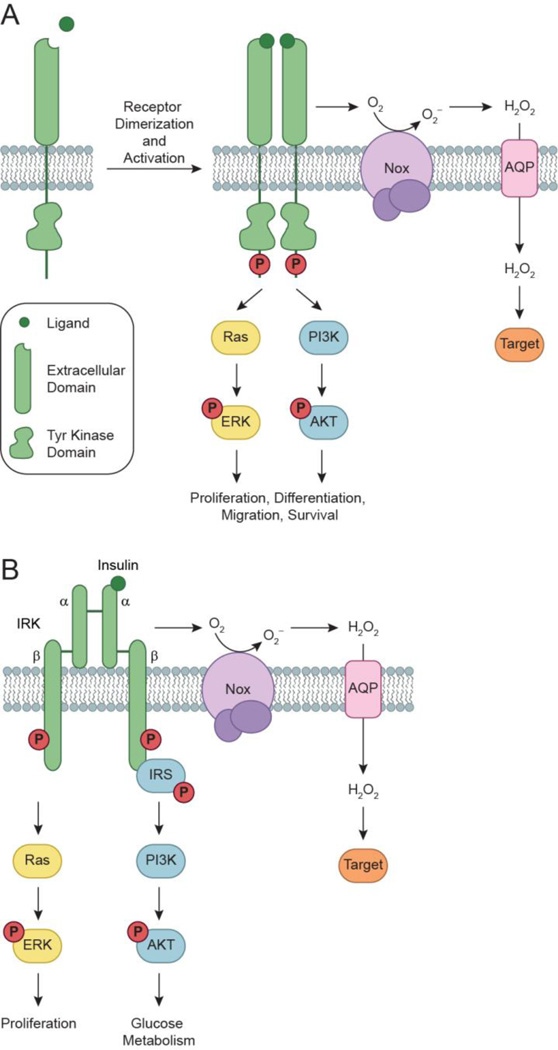
Figure 1.
Activation of RTKs and downstream signaling cascades. (a) Growth factors bind to receptor tyrosine kinases (RTKs) to induce receptor dimerization, followed by autophosphorylation of key tyrosine (Tyr) residues (red circles) located within its cytoplasmic domain. In turn, these phosphorylated Tyr residues serve as docking sites for associating proteins to activate a number of downstream signaling cascades. Two such pathways, Ras/ERK and PI3K/AKT, are shown here for simplicity. Ligand-receptor interactions also trigger the assembly and activation of NADPH oxidase (Nox) complexes, followed by subsequent production of H2O2 through spontaneous dismutation or action of superoxide dismutase (SOD). Once formed, endogenous H2O2 may pass through specific aquaporin (AQP) channels and/or diffuse across the membrane to reach the intracellular cytosol. Transient increases in H2O2 lead to the oxidation of localized redox targets. (b) Unlike other RTKs, insulin receptor kinase (IRK) exists as a heterotetrameric receptor composed of two extracellular α subunits and two transmembrane β subunits. Binding of insulin to IRK α subunits induces a conformational change in its quaternary structure to enable ATP binding, receptor autophosphorylation, and production of Nox-derived H2O2. Once activated, IRK recruits members of the insulin receptor substrate (IRS) protein family to initiate glucose metabolism through the PI3K/AKT pathway. Insulin signaling also has mitogenic effects that are mediated through Ras/ERK.