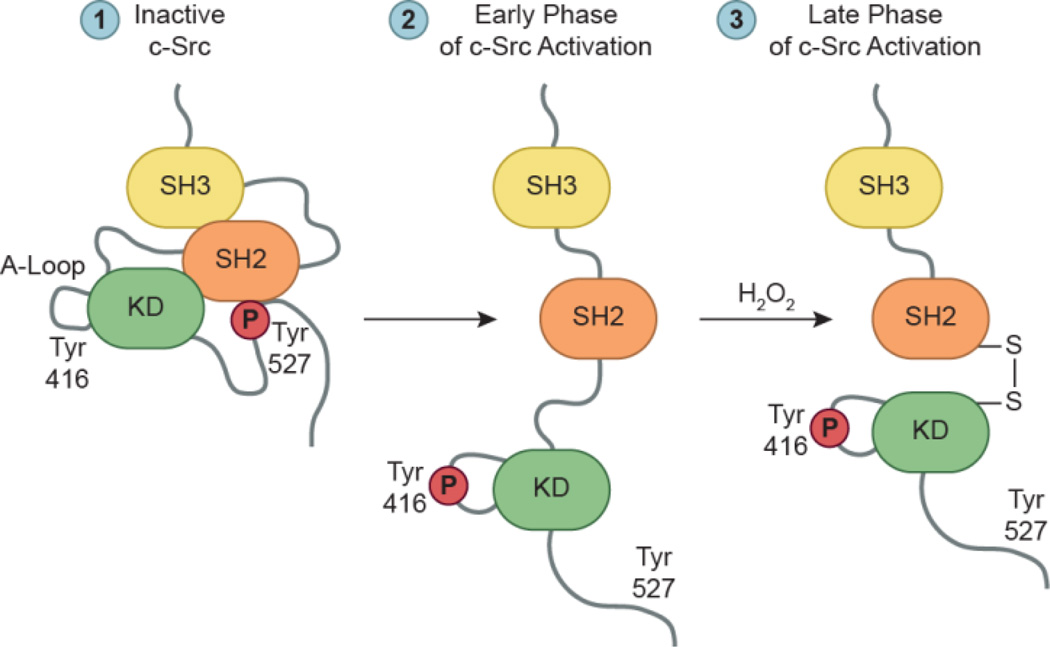
Figure 10.
Regulation of c-Src. Inactive c-Src exhibits a “closed” conformation, characterized by binding of phosphorylated Tyr527 to its SH2 domain and interactions between its SH2/SH3 domains. Growth factors and cytokines initiate c-Src activation by promoting concurrent dephosphorylation of Tyr527 and disruption of SH2/SH3 interactions to induce an “open” conformation for Tyr416 autophosphorylation. In the late phase of activation, signal-derived H2O2 mediates the formation of an intramolecular disulfide bond between c-Src Cys245 and Cys487. Bond formation promotes kinase activation, Src-mediated cell adhesion, and cytoskeletal reorganization events. Adapted from Giannoni et al., 2010.