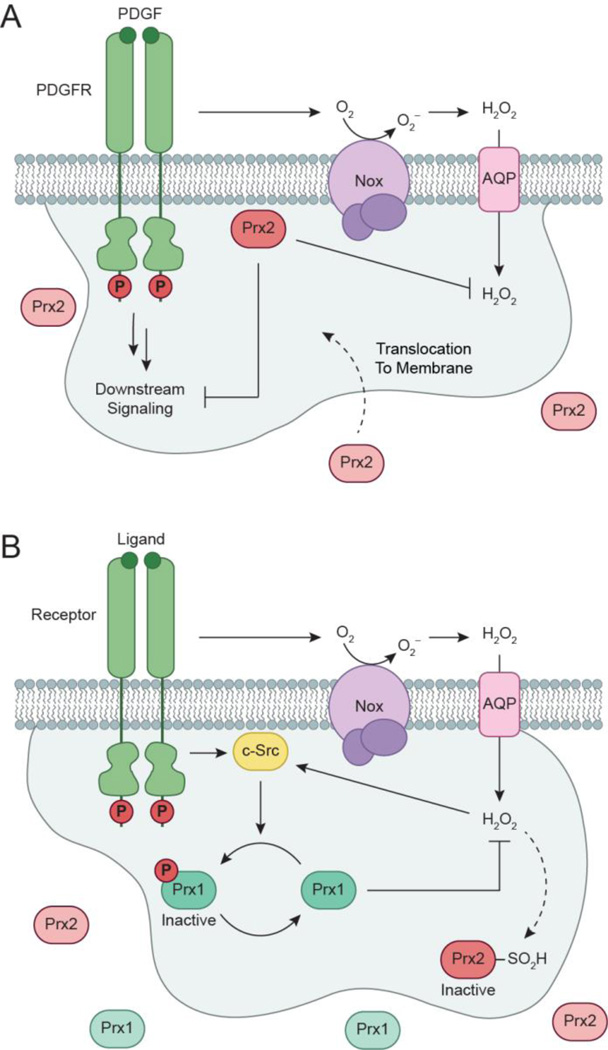
Figure 5.
Isoform-specific roles of peroxiredoxin (Prx) during redox-based PDGFR signaling. (a) PrxII functions as a negative regulator of PDGF signaling. Upon growth factor stimulation, active PrxII is recruited to the membrane and serves to relieve oxidative inactivation of membrane-associated PTPs by eliminating localized H2O2 production within the PDGFR microenvironment. (b) Receptor activation can also induce localized phosphorylation and inactivation of PrxI by PTKs, such as the redox-regulated cytoplasmic Src (c-Src). Deactivation of PrxI reduces the redox-buffering capacity adjacent to the cellular membrane, allowing for transient and localized increases in H2O2 for signal transduction. Additionally, increased H2O2 concentrations can also inactivate Prx2 by oxidation of its catalytic cysteine to sulfinic acid.