Abstract
The younger an individual starts smoking, the greater the likelihood that addiction to nicotine will develop, suggesting that neurobiological responses vary across age to the addictive component of cigarettes. Cholinergic neurons of the laterodorsal tegmental nucleus (LDT) are importantly involved in the development of addiction, however, the effects of nicotine on LDT neuronal excitability across ontogeny are unknown. Nicotinic effects on several parameters affecting LDT cells across different age groups were examined using calcium imaging and whole-cell patch clamping. Within the youngest age group (P7-P15), nicotine was found to induce larger intracellular calcium transients and inward currents. Nicotine induced a greater number of excitatory synaptic currents in the youngest animals, whereas larger amplitude inhibitory synaptic events were induced in cells from the oldest animals (P15-P34). Nicotine increased neuronal firing of cholinergic cells to a greater degree in younger animals, possibly linked to development associated differences found in nicotinic effects on action potential shape and afterhyperpolarization. We conclude that in addition to age-associated alterations of several properties expected to affect resting cell excitability, parameters affecting cell excitability are altered by nicotine differentially across ontogeny. Taken together, our data suggest that nicotine induces a larger excitatory response in cholinergic LDT neurons from the youngest animals, which could result in a greater excitatory output from these cells to target regions involved in development of addiction. Such output would be expected to be promotive of addiction; therefore, ontogenetic differences in nicotine-mediated increases in the excitability of the LDT could contribute to the differential susceptibility to nicotine addiction seen across age.
Keywords: LDT, REM sleep, electrophysiology, mouse
1. Introduction
The single largest cause of preventable death in the world is directly linked to tobacco smoking. Smoking-related diseases claim an estimated five million people each year and this figure will likely continue to grow with the increasing spread of tobacco into developing countries (Mackay and Crofton, 1996). Abstinence from smoking is hindered by development of drug dependency to nicotine, the psychobiologically-relevant compound in tobacco-containing products. Conventional medical guidelines (Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-V)) suggest that individuals must exceed a certain threshold of nicotine exposure before they can become addicted (American Psychiatric Association, 2013). Non-habitual use of nicotine in individuals with exposures below this threshold is believed to be due to a variety of psychosocial reasons distinct from neurobiological addiction. However, evidence-based clinical medicine indicates that individuals can become addicted with a much lower exposure than that suggested by the DSM-V. Indicating that neuroadaptations have occurred even in low exposure individuals, abstinence from nicotine is associated with signs of withdrawal and craving (DiFranza et al., 2011; Shiffman et al., 1995). Especially worrisome is the apparently increased proclivity of adolescences to addict to drugs of abuse. Adolescents have been shown to exhibit symptoms of addiction to nicotine within a few weeks of limited exposure such as two cigarettes a week, and withdrawal symptoms can appear in adolescents just two days after their first cigarette (DiFranza, 2008; DiFranza et al., 2000; Scragg et al., 2008). Although it is unknown whether adolescents perceive more reward from nicotine exposure than do older individuals, one possible mechanism, which is not mutually exclusive from other processes underlying their enhanced sensitivity to development of signs of addiction (Doremus-Fitzwater et al., 2010), is that nicotine is more excitatory in the young within the neurobiological reward circuitry involved in assigning a positive valiance to environmental stimuli.
The laterodorsal tegmental nucleus (LDT) is a pontine nucleus, comprised of distinct populations of cholinergic, GABAergic and glutamate containing cells, as well as neurons which co-localize ACh and GABA (Jia et al., 2003; Mieda et al., 2011; Wang and Morales, 2009). Long studied for it's role in arousal, this nucleus has recently been identified as an important component in the neurocircuitry underlying addiction-related processes (Dautan et al., 2014; Forster and Blaha, 2000; Lammel et al., 2012; Lodge and Grace, 2006; Nelson et al., 2007). The LDT sends cholinergic and glutamatergic projections to the midbrain ventral tegmental area (VTA) with a large proportion of this input synapsing on dopamine (DA)-containing cells (Omelchenko and Sesack, 2006) within this nucleus. Large effluxes of DA from VTA cells at terminals within the nucleus accumbens (NAcc) are believed to signal relevancy of behaviourally-motivating stimuli, such as drugs of abuse. Cholinergic input to the VTA, probably arising in large part from the LDT (Omelchenko and Sesack, 2005; Omelchenko and Sesack, 2006) is predominately excitatory (Mameli-Engvall et al., 2006; Melis et al., 2013), acting at nicotinic acetylcholine receptors (nAChRs) and muscarinic acetylcholine receptors (mAChRs) demonstrated on DA-containing cells (Miller and Blaha, 2005; Pidoplichko et al., 2004). Supporting the conclusion that the LDT-VTA pathway is functionally important, inactivation of the LDT prohibits the DA-containing VTA neurons from burst firing (Lodge and Grace, 2006), a firing pattern required for behaviorally relevant release of DA from these cells (Floresco et al., 2003; Grace and Onn, 1989). Furthermore, optogenetic stimulation of the LDT-VTA pathway is sufficient to induce addictive-related behaviour in mice, indicating that stimulation of LDT neurons can prompt reward, possibly involving glutamate release in the VTA (Lammel et al., 2012). These and other studies (Forster and Blaha, 2000) indicate that the LDT plays an important role in addiction processes. Accordingly, it is important to understand how drugs of abuse, such as nicotine, affect cholinergic and glutamatergic cellular activity within the LDT, which would be expected to affect output to target midbrain reward-related nuclei.
Based on the clinical data showing a heightened sensitivity of adolescents to addict to nicotine and the role of the LDT in the reward circuitry, we hypothesized that actions of nicotine on cholinergic LDT neurons varies across ontogeny, resulting in a differential response to nicotine in younger individuals from responses elicited in LDT neurons from older animals. Although we have previously shown that nicotine and other nAChR agonists have strong actions on the membrane excitability of cholinergic and non-cholinergic LDT neurons via both pre- and postsynaptic mechanisms (Ishibashi et al., 2009), we did not examine age-related differential effects of this drug on LDT cellular activity. Accordingly, in the present study, we examined our hypothesis of an age-related difference in nicotinic actions using in vitro calcium imaging and patch clamp electrophysiology to compare nicotine-induced actions on cholinergic and non-cholinergic LDT neurons across different ranges of postnatal ages. Additionally, we examined ontogenetic changes in nicotine-induced synaptic input to cholinergic and non-cholinergic LDT neurons. Taken together, our data indicate that age-associated nicotinic activation of cholinergic LDT neurons could result in a greater excitation of DA-containing VTA cells in younger animals and, in conjunction with known direct activation by nicotine of DA-VTA neurons, contribute to the processes of assigning reward to this drug of abuse differentially across ontogeny.
2. Methods
2.01 Animals
These studies were performed in mice across different developmental stages. The gestational period of a mouse is roughly equivalent to the first and second trimesters of human development and the first week of postnatal life corresponds to the third trimester of human gestation (Bayer et al., 1993; Dobbing and Sands, 1979). All animal studies complied with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and with Danish laws regulating experiments on animals. After determining that efforts to reduce the number of animals used, to explore alternatives to animal experiments and to minimize animal suffering had been made, the animal studies were permitted by the Animal Welfare Committee, appointed by the Danish Ministry of Justice.
2.02 Tissue preparation
NMRI (Taconic, Denmark or Harlan, The Netherlands) or C57 mice (Taconic, New York) (age 7–34 days) were anaesthetized with isofluorane and decapitated. A block of the brain containing the LDT was extracted into ice-cold artificial cerebrospinal fluid (ACSF) (in mM: NaCl 124, KCl 5, Na2HPO4 1.2, CaCl2 2.7, MgSO4 (anhydrous) 1.2, Dextrose 10, NaHCO3 26, oxygenated in 95% oxygen/5% carbogen) and subsequently mounted into the cutting chamber of a Leica vibrotome (VT1200S). Coronal slices (250μm) were cut in ice-cold oxygenated ACSF and subsequently incubated for 15 min at 37°C. Recordings were conducted at room temperature.
2.03 Calcium imaging
As a high yield, rapid screen for nicotine-induced cellular activity across different developmental ages, we used “bulk-loading” calcium imaging which allows monitoring of large numbers of cells (Fig 1 A) without complete change of the cytoplasm, including second messengers that may be important in the mediation of responses following drug exposures. Since this is a high yield method, we were able to monitor adequate numbers of cells to allow examination of age-related changes in nicotine-induced intracellular calcium changes across three relatively narrow age groups. To this end, we incubated slices from three different age groups (group A, postnatal (P)8-P10; group B, P11-P15; group C P16-P21) with the calcium indicator dye, fura 2-AM (15 μM in DMSO; Molecular probes, Invitrogen, Denmark), which crosses cellular membranes and following de-esterification, becomes trapped intracellularly (Tsien, 1981). Within the LDT, this calcium binding dye allows measurements of changes in intracellular calcium concentrations as, when excited by the appropriate wavelength, the dye alters its emission spectra when calcium is bound (Kohlmeier et al., 2004). Brain slices were incubated in oxygenated dye at 31°C for 10 minutes plus one minute for every day of age. Using this method of dye loading, the quality of the fura 2-AM loading of LDT neurons decreases dramatically after three weeks of postnatal age. Therefore, only NMRI mice aged P7-P21 days were utilized. After the appropriate time in the dye solution, the slice was placed in the recording chamber and rinsed for 30 minutes to remove excess dye before optical recordings began. The LDT was located using a 4x objective and neurons were visualized under differential interference contrast optics with a 40x water immersion objective (NA 0.8, Olympus, Germany), mounted on a fixed stage microscope (Olympus BX50WI, Germany). Neurons were visualized and fluorescent measurements were made using a frame-transfer, cooled 12 bit CCD camera system (Sensicam, PCO Instruments, Germany) controlled by TILL-VISION software (Till Photonics, Germany) and an xenon light source (Osram, Germany). Initially, a high-resolution, full frame image was acquired. Regions of interest (ROI) within this full frame image were selected to encompass dye loaded cells. For data collection, each pixel within this ROI was summed according to binning parameters best selected to balance the spatial and temporal resolution (2 × 2). Fura 2-AM is a ratiometric dye and therefore fluorescence changes were measured using excitation at two different wavelengths (340 and 380 nm) and values obtained were ratioed (F = F340/F380) following subtraction of autofluorescence, which was determined from a ROI selected in the field where dye-filled cells were not apparent at any level of focus. Temporal and spatial changes are presented using the equation dF/F. In this equation, the difference in fluorescence at rest is subtracted from the maximum change in fluorescence (dF), which is then divided by the fluorescence at rest (F) with upward going deflections indirectly indicating rises in calcium. Exposure times for each wavelength were selected so that the brightest pixel in the field was between 7-10 % of the upper limit of the dynamic range which reduced bleaching of the dye and phototoxicity due to overexposure. Dye loading was found to vary from slice to slice, as well as from cell to cell. To determine whether drug-induced changes were due to poorer loading in older animals, the baseline fluorescence of both wavelengths was examined and found to differ across the three age groups with a higher intensity in the older groups (340nm group A, n= 427, F= 106.8 ± 2.2; group B, n= 396, F= 142.4 ± 2.6; group C, n= 340, F= 131.7 ± 2.6; and 380nm group A, n= 387, F= 139 ± 3.3; group B, n= 390, F= 182 ± 3.3; group C, n= 339, F= 165.6 ± 3.6; one way ANOVA p < 0.05). However, by measuring relative changes in fluorescence normalized in each cell from baseline, rather than absolute changes, variations in dye loading from slice to slice should not have biased the analysis. In addition, to examine whether poorer dye loading or cell health compromised our data, we examined the calcium rises induced by NMDA (10 μM) across different postnatal ages, and found that while larger rises in calcium were induced in slices from the older age group, there was no significant difference in dF/F across the range of ages used in the present study (P7-15, dF/F% = 139.3, n= 18; P16-21: dF/F% = 150.2, n= 18; two way t-test p> 0.05).
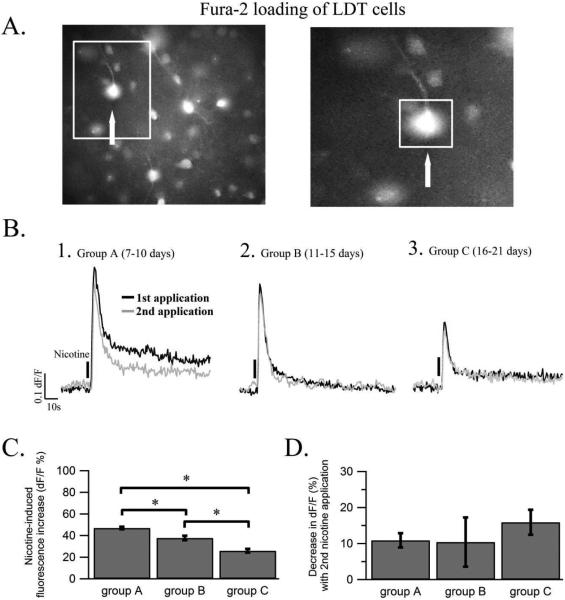
Figure 1.
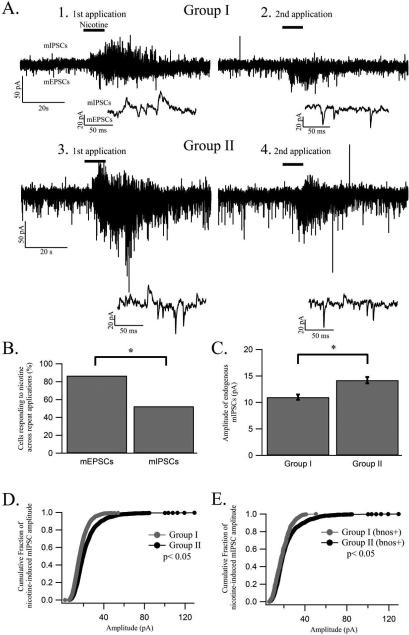
Nicotine elicits greater changes in fluorescence indicating larger rises in intracellular calcium in LDT neurons of animals from younger age groups. (A) In the left panel an image of fura-2 loaded cells (F380 nm) within the LDT, in which changes in fluorescence were examined, is shown. The white box indicates the region expanded in the right panel and the arrows indicates the same cell in both panels. The region of interest from which fluorescence was monitored is indicated by a square around the cell soma in the right panel. (B) Representative ΔF/F traces are shown of the changes in fluorescence induced by an initial (black) and a second (grey) nicotine application in a LDT cell from group A (B1), group B (B2) and group C (B3) indicating rises in intracellular calcium. The nicotine-induced change in ΔF/F is greatest in the youngest group A when compared to that elicited in the older group B and group C. The repeat application of nicotine ten minutes after the initial nicotine application induced a smaller increase in fluorescence in all three age groups, but was still higher in young animals. Nicotine application is marked by a vertical line. (C) Histogram showing the mean nicotine-induced increase in fluorescence in percent elicited in cells from each of the three age groups. The average amplitude found in group A was significantly larger than that of group B and group C. Further, in group B the average amplitude was larger than that of group C. * denotes a significance level of p< 0.05 in this and subsequent figures. (D) The percentage decrease in ΔF/F of the second compared to the first nicotine application for group A, group B and group C are shown in the histogram. There was no significant difference in the percentage decrease in ΔF/F between the three groups in response to a repeat nicotine application.
2.04 Whole-cell patch clamping
Whole-cell patch clamp electrophysiological recordings were performed using thin walled borosilicate electrodes (3-7 MΩ) fashioned from a Sutter P-97 horizontal puller (Sutter instruments, USA). Electrodes were filled with a recording solution containing (in mM) K-gluconate 144, KCl 2, HEPES 10, EGTA (tetraacetic acid) 0.2, Mg-ATP 5, Na-GTP 0.3. This patch solution contains a low chloride concentration, which increases the driving force for chloride (chloride reversal potential: −107 mV at 22°C) allowing easier detection in the same recording of both spontaneous excitatory postsynaptic currents (sEPSCs; downward going deflections) and spontaneous inhibitory postsynaptic currents (sIPSCs; upward going deflections) and miniature EPSCs (mEPSCs) and miniature IPSCs (mIPSCs) when recordings were performed while action potentials were blocked. Recordings containing both inhibitory and excitatory events allowed us to compare nicotine actions on both types of events within the same cell. To post-hoc identify cells immunohistochemically, biotinylated Alexa-594 (25 μM, Molecular Probes, Invitrogen, Denmark) was also included in the patch solution and allowed to passively diffuse into the cells during recording.
Whole-cell patch clamp recordings were performed under visual guidance using the same camera system utilized for optical recordings. A high resistance seal (giga) between the pipette and the cell membrane was obtained using a patch clamp EPC9 amplifier (HEKA, Germany) operated in voltage clamp mode using Pulse version 8.8 software (HEKA, Germany). Current traces were recorded using AxoScope 10.2 (Molecular devices corporation, USA) and an Axon CNS Digidata 1440A digitizer (molecular devices corporation, USA) at a sampling frequency of 10 kHz. Recordings were uncorrected for the liquid junction potential, which was estimated to be approximately 15 mV, and discarded if the holding current exceeded 50 pA or the series resistance exceeded 30 MΩ. Tissue was continuously perfused with oxygenated ACSF at room temperature at a rate of approximately 3 ml/min. Although cell diameter is not an absolute indicator of cell phenotype for LDT neurons, cholinergic cells are usually larger than GABA-containing cells in this nucleus (Boucetta and Jones, 2009; Wang and Morales, 2009); however, evidence for co-localization of GABA and acetylcholine (ACh) in at least a subset of a population of LDT cells has been presented (Jia et al., 2003; Mieda et al., 2011). Regardless, to optimize recordings from cholinergic neurons, we targeted larger multipolar neurons for recordings.
Large decreases in nicotinic binding sites from P7 to P21 have been found in mice within the brainstem (Zhang et al., 1998) where the LDT is located and cholinergic LDT cell have been reported to assume a more adult like profile around P15 (Ninomiya et al., 2005). Accordingly, to examine developmental differences in nicotine-induced responses, we divided the recorded cells into two age groups. Group I containing animals at an age reported to exhibit the highest number of nicotinic binding sites (P7-P15) and the second group, group II, containing older animals which showed a decrease in numbers of nicotinic binding sites (P16-P34 day). We did test, in preliminary studies, that further subdivision of the age groups did not yield an apparent difference.
2.05 Drugs
Nicotine ((−)-nicotine ditartrate, Tocris, UK) was prepared freshly each day from frozen aliquots under minimum light exposure. For electrophysiological experiments, nicotine was applied utilizing several application methods. Nicotine was puff applied with a picospritzer III (Parker Hannifin corporation, USA) with a pressure of 15-20 PSI using borosilicate micropipettes (3-7 MΩ). The application pipette was positioned just above tissue approximately 60-80 µm upstream from the recorded cell. This effected a relatively immediate and local nicotine application. In other recordings nicotine was either bath applied through the perfusion ACSF solution or applied via pipette application directly into the recording chamber. During calcium imaging recordings, to minimize artifacts due to tissue movement, the application pipette was located at a greater distance above tissue and nicotine was picospritzed with a lower pressure of 6-10 PSI. To facilitate maximum nicotine exposure across the entire visible tissue area, the application pipet was located at the edge of the field of view and a nicotine concentration of 100 mM was applied. In these experiments, a second application of nicotine was applied 10 minutes after the initial application and in a subset of cells nicotine was applied a third time 20 minutes after the second application in the presence of tetrodotoxin (TTX, Alamon, Israel, 500 nM) to block action potential generation. TTX was dissolved in ACSF from frozen aliquots and bath applied. In some recordings 6,7-Dinitroquinoxaline-2,3(1H,4H)-dione (DNQX, 15 μM, Sigma), D(−)-2-Amino-5-phosphonopentanoic acid (AP5, 50 μM, Sigma), SR-95531 (Gabazine (GZ), 20 μM, Sigma) and strychnine (2.5 μM, Tocris, UK) were used to block AMPA and kainate, NMDA, GABA and glycine receptors respectively, to eliminate effects of presynaptic excitatory and inhibitory neurotransmitter release. Aliquots of the nAChR antagonists dihydro-β-erythroidine hydrobromide (DHβE, 500 nM, Tocris, UK) and methyllycaconitine citrate (MLA, 10 nM, Tocris, UK) were dissolved in ACSF containing, TTX, DNQX, AP5, GZ and atropine (5 μM, sigma, USA). The antagonists were applied to the recording chamber via the perfusion ACSF for at least 5 minutes before responses to acetylcholine (ACh, acetylcholine chloride, 1mM, Sigma, USA) were monitored. ACh was used as the cholinergic agonist for the nAChR subunit antagonist study as it had been determined that significant attenuation was not present upon repeat applications (Ishibashi et al., 2009).
2.06 Protocols
The cellular membrane potential was maintained at −60 mV and in whole-cell voltage clamp mode hyperpolarization activated cationic (Ih) currents were elicited by application of 500 ms duration hyperpolarizing steps to −100 mV and −110 mV as preliminary studies revealed that Ih currents were clearly visible at hyperpolarization steps at, and more negatively than −100 mV, whereas Ih currents were less apparent at hyperpolarization steps more reduced than −100 mV. The amplitude of the Ih current was measured as the difference between the minimum current required to reach the set voltage and the current level measured at the end of the hyperpolarization step. Input resistance (Ri) was calculated from the amount of current necessary to step the voltage from rest to −70 mV for 100 ms.
Action potentials were recorded in current clamp mode and elicited by injecting current for a duration of 100ms – 500ms to induce a single action potential. Action potential parameters were measured on isolated action potentials using custom designed macros for Igor Pro software (Wavemetrics, USA). The action potential firing threshold was measured as the potential at which the second derivative of the voltage waveform exceeded three times its standard deviation in the period previous to the onset of the spike. The spike width1/2max was measured at half the maximum spike amplitude and the spike amplitude was measured as the difference between the firing threshold and the spike peak. The maximum slope of the rise to the action potential peak and decay to baseline were measured as the maximum and minimum of the smoothed first derivative of the voltage waveform. The afterhyperpolarization (AHP) amplitude was measured as the difference between the firing threshold and the minimum value of the AHP (AHP minimum). To examine cellular firing rates, current pulses of increasing intensities were applied for a duration of 1000 ms until firing failure occurred or the maximum applicable current (999 pA) was reached. Current pulses were analyzed until the maximum number of action potentials was reached and the steady state firing frequency was calculated by measuring the frequency of the last 7 spikes using custom designed macros for Igor Pro software.
2.07 Analysis of data
Although differences in neuronal responses to nicotine might exist between mouse strains, preliminary studies suggested there were no apparent differences between the two strains used and no division was therefore made based upon strain. A subset of animals used in the electrophysiological studies had been prenatally exposed to saccharine for a different series of examinations, but as the results obtained from these and naïve mice revealed no detectable differences, data were pooled. All figures were made using Pro Igor software. Amplitudes of membrane currents (exceeding 3 pA) were measured as the difference between the current at baseline and at the peak of drug effect, taking care that regions selected did not include synaptic activity, and values were determined by an average of 5000 points. In addition, recordings were conducted with blockade of glutamate, GABAA and voltage-dependent sodium channels to confirm that averages were not reflective of inclusion of synaptic events. Measurements of the number and amplitude of synaptic currents (PSCs) were performed using MiniAnalysis (Synaptosoft, USA) with a measurement period of 30s before (control) and after nicotine application and inhibitory and excitatory PSCs were analyzed from the same record. Simultaneous recordings were necessary to examine the functional outcome of presence of both the inhibitory and excitatory events, one caveat with our analysis was that small amplitude events may not have been detected, especially if inhibitory and excitatory events co-occurred. Kolmogorov-Smirnov (KS-test) statistics was used to examine for a significant difference in the cumulative distributions of PSCs between control and drug period in individual cells. Comparisons between groups were done using t-tests, Chi Square (χ2)-test, KS-tests, and ANOVAs with a significances level of 0.05 and data is offered as mean ± standard error of mean.
2.08 Immunohistochemistry
Although choline acetyltransferase is a well-known marker for cholinergic cells, in our experience achieving successful stain for this enzyme in the LDT from brain slices which have been suspended in ACSF for the duration of a recording period has proven difficult. Staining in brain slices from which electrophysiology has been performed results in a low signal to noise ratio, precluding confident phenotypical identification. LDT neurons can, however, be identified as either cholinergic or non-cholinergic by presence or absence of brain nitric oxide synthase (bnos), which is a known and reliable marker for cholinergic neurons in this nucleus (Vincent and Kimura, 1992; Vincent et al., 1983). Additionally, staining for this marker is robust in brain slices collected from brains which have not been perfused, and which have been subject to hours of electrophysiology. Accordingly, to confidently and unambiguously identify LDT neurons as cholinergic, we performed immunohistochemistry to detect the presence of bnos in recorded cells. Accordingly, slices were fixated in 4 % paraformaldehyde for a minimum of four hours and then stored in 30 % sucrose in phosphate buffer saline solution for a minimum of 24 hours. Slices were resectioned to a thickness of 40 μm on a cryostat (Leica CM 3050S) and incubated in an anti-bnos antibody (rabbit polyclonal, cat# N7280, Sigma-Aldrich, Denmark). Following overnight incubation at room temperature in the primary antibody, the slices were transferred to a fluorescent secondary antibody (anti-rabbit, goat, cat# A11008, Molecular probes, Denmark). Recorded cells were detected by optics optimized for visualization of the Alexa 594, which had passively diffused into the cell from the patch pipette. Identification of the Alexa 594 filled cell as cholinergic or non-cholinergic was done by detection of the co-presence, or absence, of the secondary antibody when viewed under appropriate optics (see Fig 2 A).
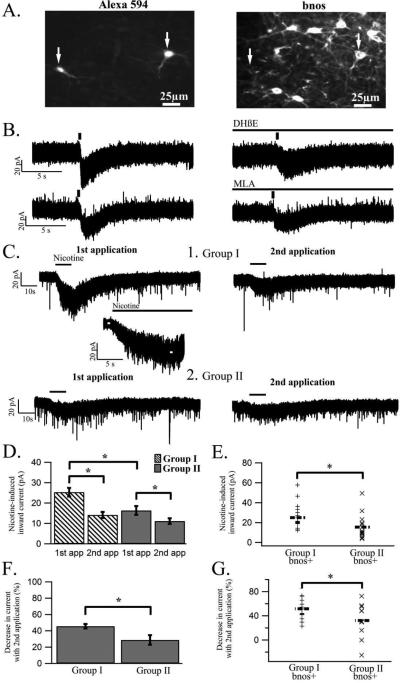
Figure 2.
Nicotine induces larger inward currents in P7-P15 as compared to P16-P34 animals along with a larger reduction in current in response to repeat nicotine applications in identified cholinergic LDT cells. (A) In the present study the phenotype of electrophysiologically recorded cells was determined as either cholinergic or non-cholinergic. Due to the presence of the fluorophore Alexa-594 in the patch solution, recorded cells were visible using appropriate fluorescence microscopy (arrows, left panel), and immunohistochemical staining with bnos antibody (right panel) enabled identification of recorded cells as cholinergic based on the presence of both bnos and Alexa-594 (right arrow in both panels) or non-cholinergic based on the absence of bnos in the recorded cell (left arrow in both panels). (B) The top left trace is a representative whole-cell, voltage clamp recording from a group II LDT cell showing the inward current induced by puff application of 1mM of acetylcholine (ACh) for 0.3s which is marked by a vertical line. In the top right trace, bath application of DHβE to the same cell attenuated the ACh-induced inward current indicating presence of nAChRs containing the β2 and/or β4-subunit in LDT neurons. In the two bottom traces, bath application of MLA is shown to decrease the ACh-induced inward current in a cell from group I, indicating the presence of α7-containing nAChRs. The applied antagonists were found to decrease the ACh-induced current in both age groups indicating the presence of α7- and β2 and/or β4-subunits across both examined age groups. (C1) The left panel is a representative whole-cell, voltage clamp recording of a cholinergic LDT cell from group I showing an inward current induced by puff application of 10 μM nicotine (application indicated by black line). Below the left panel is an expanded inset of the nicotine-induced current in which the white lines on the recording trace represents the areas from which the current amplitude was measured. In the right panel the inward current induced by a second application of nicotine in the same cell is shown. As was found in the majority of cells, the inward current evoked by the first application of nicotine was larger than that of the second application of nicotine. (C2) Whole-cell recording from a group II cholinergic LDT cell showing nicotine-induced inward currents of the first nicotine application on the left side and the second application of nicotine resulting in a smaller inward current on the right. (D) Histogram from the entire population of cells indicating that the inward current induced by the first application of nicotine is significantly larger in group I as compared to group II, while no difference is seen between the two groups for the repeat nicotine application. Also apparent in both group I and group II is a significant decrease in the nicotine-induced inward current of the repeat compared to the initial nicotine application. (E) Scatter plot showing the nicotine-mediated inward current in individual cholinergic LDT neurons from group I and group II. The nicotine-induced inward current is significantly larger in cells from group I as compared to group II. The dotted line in each column indicates the mean value. (F) The percentage decrease in the inward current induced by the repeat compared to the initial nicotine application for group I and group II is shown in a histogram. A significantly larger decrease in the inward current in response to a second nicotine application is seen in group I as compared to group II. (G) Scatter plot showing the percentage decrease of the initial compared to the repeat nicotine-induced inward current in cholinergic LDT neurons from group I and group II, indicating that across this population of cells a significantly larger decrease in the inward current in response to a second nicotine application is seen in group I as compared to group II. Within each column the dotted line indicates the mean value.
3. Results
3.01 Nicotine induces larger rises in intracellular calcium in LDT neurons from animals belonging to younger as compared to older age groups
As an initial step in detecting possible developmental-related changes in nicotine-induced responses of LDT neurons, we investigated differences in nicotine-induced rises in calcium across three age groups, group A (P7-P10), group B (P11-P15) and group C (P16-P21). We used “bulk-load” calcium imaging (Fig 1 A), in which calcium changes could be monitored across a large population of LDT neurons in the same slice without disturbing the intracellular contents that may be involved in nicotinic responses (Kohlmeier et al., 2004). Following loading of LDT-containing brain slices with the ratiometric dye, fura 2-AM, we found that a significantly larger number of LDT cells responded with an increase in dF/F, indicating a rise in intracellular calcium in response to puff application of nicotine (100mM, 2s) in the youngest as compared to the older age groups (group A: 94.5 % responded, n= 329; group B: 89.72 % responded, n= 360; group C: 84.71% responded, n= 344; Chi Square (χ2)-test). In addition to eliciting calcium responses in a larger number of cells, the amplitude of calcium rises induced in group A was 24 % and 80.9 % larger than that seen in group B and group C, respectively, which was a significant difference (one-way ANOVA; Fig 1 B and C). In addition, the change in fluorescence in group B was significantly larger than that elicited in group C (45.9 % larger, one-way ANOVA; Fig 1 B and C). In the presence of TTX, the average amplitude of nicotine-induced rises in calcium was significantly larger in neurons from the youngest age group when compared to rises seen in neurons from the two older age groups (group A, n= 109; group B, n= 26; group C, n= 21; one-way ANOVA). This indicates that the differences observed in the nicotine-induced intracellular rises in calcium between the different age groups are not dependent upon voltage-dependent sodium channels or action potential generation within the slice.
Since calcium can enter LDT cells through nAChR dependent mechanisms and nAChRs have been shown to differentially desensitize depending upon their specific subunit composition (Quick and Lester, 2002; Wooltorton et al., 2003), which could vary across development (Zhang et al., 1998), we examined the effects of a repeat nicotine application. Following recovery back to baseline, in the majority of cells, second applications of nicotine resulted in smaller rises in intracellular calcium from those elicited in first applications, which was significantly different across all three groups (two-tailed paired t-test), however, the attenuation did not significantly differ across ontogeny (group A: 10.9 ± 2.64 % reduction; group B: 10.42 ± 4.09 % reduction; group C: 15.93 ± 3.47 % reduction; one-way ANOVA; Fig 1 D). Interestingly, in a subset of cells, a larger rise in calcium was elicited by a second nicotine application as compared to the first nicotine application and the increase was in some cases more than two-fold. The increased calcium response following a second application of nicotine could be due to nicotine-induced desensitization of nAChRs located on local GABAergic neurons following first applications leading to larger calcium rises in postsynaptic cells upon subsequent nicotine exposure. However, elucidation of the underlying mechanism(s) behind this increase was beyond the scope of this study since the phenomenon showed no association with age (χ2-test p> 0.05).
Taken together, our calcium imaging data demonstrate that in animals from the younger age groups, nicotine induces an increase in intracellular calcium in a larger proportion of LDT cells as compared to that induced in neurons from animals belonging to older age groups. Furthermore, the increase in calcium in the younger animals is larger than that seen in older animals and does not rely on sodium channel dependent action potential generation in the slice, which indicates that calcium dependent functions will be more affected by nicotine exposure in LDT cells from younger mice. While nicotine induced increases in intracellular calcium can arise via a variety of differing mechanisms, likely one source of nicotine-induced calcium in LDT cells is calcium influx via calcium permeable nAChRs (Ishibashi et al., 2009; Shen and Yakel, 2009). Accordingly, our data suggest the possibility of age-related changes in number, and/or properties of nAChRs, which extends to nAChR receptors located postsynaptically, and/or on terminals directed to, imaged LDT cells.
3.02 Nicotine induces a larger inward current in cholinergic LDT neurons from P7-P15 as compared to P15-P34 animals
To investigate whether nAChRs demonstrated to be located postsynaptically on LDT neurons (Ishibashi et al., 2009) exhibit functional differences across ontogeny, we performed whole-cell, voltage clamp recordings. We compared nicotine-induced inward current between identified cholinergic and non-cholinergic LDT neurons (Fig 2 A) across two different age groups, group I (P7-P15) and group II (P16-P34). Preliminary studies showed that division of group I into two age groups (P7-P10 and P11-P15) revealed no significant difference between those groups and therefore cells from ages P7-P15 were pooled. We have previously shown that brief, local application of nicotine elicits postsynaptic responses (Ishibashi et al., 2009) and that these responses were mediated by activation of nAChRs containing α7 and β2 and/or β4 nAChR subunits. However, that study did not discriminate across different ages. Accordingly, we verified that nAChRs on LDT neurons from the age groups utilized contained these subunits. Control responses to cholinergic stimulation were compared to repeat responses in presence of either MLA or DHβE, which are relative specific antagonists for α7 and β2 and/or β4 nAChR subunits (Alkondon and Albuquerque, 1993; Alkondon et al., 1992), respectively, and it was found that both antagonists attenuated nAChR-mediated inward currents (Fig 2 B) in both group I and group II, thereby confirming the presence of postsynaptic α7- and β2 and/or β4-containing nAChR subunits in both age groups. Subsequently, under visual guidance, low concentrations of nicotine (10 M nicotine, 10s puff) were picospritzed locally onto the recorded cell, in order to facilitate postsynaptic responses and to reduce the extent of nicotine exposure. In addition, picospitzing was utilized to reduce receptor desensitization before peak current amplitudes were achieved. The number of cells responding with inward currents upon nicotine application was significantly greater in P7-P15 animals than the number responding in P16-P34 animals. In LDT neurons from group II, 78 % (39/50) of the recorded cells responded to nicotine with an inward current; whereas, 97.1 % (67/69) of cells responded with inward currents in group I (χ2-test p< 0.05). Further demonstrating ontogenetic differences in nicotine responsitivity within the LDT, the amplitude of the nicotine-induced inward current was greater in responding cells from group I than that induced in cells from group II (35.5 % larger, two-tailed t-test p< 0.05; Fig 2 C and D). In a subpopulation of cells identified as bnos positive, the amplitude of the nicotine-induced inward current was significantly greater in group I when compared to that elicited in group II (38.2 % larger, group I n= 19; group II n= 15; two-tailed t-test; Fig 2 E). In bnos negative cells, no significant difference was found in the amplitude of nicotine-induced current across age (group I n= 10; group II n= 11; two-tailed t-test). To verify that differences in current amplitude were not due to contamination of the record by synaptic events, a subpopulation of cells was recorded in the presence of glutamate and GABAA receptor antagonists as well as a blocker of synaptic transmission (DNQX, AP5 GZ, strychnine and TTX, respectively). As the nicotine-induced current amplitude in the presence of blockers was not significantly different in absence of synaptic blockade (group I, 30.85 ± 6.9 pA, n=6; group II, 7.23 ± 1.1 pA, n=4; t-test p> 0.05), and the induced current amplitude was significantly different (two way t-test) across the two age groups in the presence of blockers, we conclude that membrane inward currents stimulated by nAChRs located postsynaptically on LDT cells show an age related reduction in amplitude.
Differential desensitization kinetics of nAChRs could occur as a result of developmental changes in nAChR subunit composition between the two age groups examined which could lead to differences in responses upon repeat nicotine exposure. Therefore, we examined the effect of second applications of nicotine following a recovery period of 5 minutes after the first application. In group I (n= 57), the nicotine-induced inward current was 45.7 ± 2.7 % smaller than that elicited by the first nicotine application, and the attenuation of nicotine-induced currents upon second applications was significantly larger when compared to that of group II (28.8 ± 5.8 % reduction, n= 30; two-tailed t-test; Fig 2 C and F). The larger reduction in amplitude of nicotine-induced currents in group I extended to a population of identified bnos positive cells (group I: 52.2 ± 4 % reduction (n= 17); group II: 33.2 ± 8.9 % reduction (n= 10); two-tailed t-test p< 0.05; Fig 2 G). In bnos negative cells, there was no significant difference in response to a second application of nicotine across the two age groups (group I: 38.7 ± 12.3 % reduction, n= 8; group II: 21.8 ± 12.9 % reduction, n= 9; two-tailed t-test).
The results indicate an age-related difference in the effects of nicotine on LDT neurons with a larger number of cells in this nucleus responding with inward currents in the younger age group. Further, the data suggest that the nicotine-mediated response amplitudes differ in cholinergic LDT neurons across different ontogenetic stages with greater reductions in current amplitudes induced by second applications in the younger age group. Larger initial responses in the younger age group could reflect increases in numbers of nAChRs and/or shifts in subunit compositions of functional receptors across age. Reductions of the amplitude of nicotine-induced currents upon repeat exposures could be due to receptor desensitization, which is highly dependent upon the particular composition of subunits comprising the nAChRs (Quick and Lester, 2002; Wooltorton et al., 2003). Accordingly, when taken together, our study examining nicotine-induced current amplitudes and repeatability of effects support the possibility of a developmental shift in the presence of subunits comprising nAChRs on cholinergic LDT neurons across age.
3.03 Developmental- and nicotine-induced decreases of the input resistance (Ri) of LDT neurons
As the intrinsic membrane properties of neurons can have profound effects on cellular excitability, our next step was to examine whether nicotine had actions on these properties in neurons within the LDT that were differential across age. However, as it has been well documented that cellular membrane properties alter across age in other brain regions where it has been studied, as a first step we had to determine whether differences in these properties existed in LDT neurons across the range of ages in our study. Our first examination focused on Ri, which when using the whole-cell patch clamp technique reflects in large part the degree of somatic conductances. Although we have previously reported no difference in Ri across cells from animals ranging in age from P12 to P28 (Kristensen et al., 2007) in the present study, we found that the Ri was 26.3% lower in LDT neurons from group II when compared to that from group I, which was a significant difference (group I: 1068.4 ± 58.7 MΩ, n= 47; group II: 787.1 ± 36.7 MΩ, n= 64; two-tailed t-test). While an insufficient number of bnos negative cells were recovered to enable comparison across age, a significantly lower Ri was detected in bnos-positive LDT neurons from group II when compared to the Ri measured in a bnos-positive population of cells from group I (group I: 1100.7 ± 75.8 MΩ, n= 35; group II: 792.2 ± 47.5 MΩ, n= 40; two-tailed t-test). Our detection of a difference in Ri across age, which is in contrast to our earlier findings (Kristensen et al., 2007), likely reflects inclusion in the present study of cells from animals at a younger postnatal age. This suggests that significant developmental changes in Ri could occur between P7-P12, which has also been seen in other brain regions (McCormick and Prince, 1987; Ramoa and McCormick, 1994). A greater Ri in cells from P7-P15 animals suggests that excitatory and inhibitory currents directed to these cells would result in larger changes in membrane potential, which is interesting in light of findings that nicotine activates excitatory and inhibitory presynaptic inputs in the LDT (Ishibashi et al., 2009).
After establishing age-related differences in cellular Ri, nicotine was applied (500nM, bath application) and found to significantly decrease Ri in cells from both group I and group II (one-tailed paired t-test), however, the nicotine-induced decrease was not significantly different between the two groups (group I: 26.1 ± 6.3 % decrease, n= 11; group II: 21.8 ± 5 % decrease, n= 9; two-tailed t-test). In cells identified as bnos positive, nicotine likewise significantly decreased the Ri in both groups (one-tailed t-test) but did not differentially decrease the Ri across the two group (group I: 20.2 ± 8 % decrease (n= 5); group II: 27.8 ± 6.2 % decrease (n= 6); two-tailed t-test p> 0.05). In 8 cells across the two age groups, a partial recovery of the Ri following washout was recorded (66.6 ± 13.9 % recovery) indicating drug-application associated changes were not due to deteriorating cell conditions.
Our data corroborate findings from a previous study showing a nicotine-induced decrease of the Ri (Ishibashi et al., 2009). However, since the baseline Ri was higher in the younger age group, the same relative nicotine-induced change across the two age groups suggests the possibility that there are differences in the number of nicotine-activated channels with a reduced channel number in animals from the younger age group. If this is the case, taken in combination with our findings of a larger nicotine-induced current in group I animals, this suggests that high affinity nAChRs contribute to the nicotine-induced inward current to a larger extent in P7-P15 compared to P16-P34 animals.
3.04 Developmental- and nicotine-induced effects on the hyperpolarization activated cationic (Ih) current
Cholinergic LDT neurons possess a prominent Ih current, which in response to hyperpolarizations significantly more negative than resting membrane potential, can induce a strong depolarizing effect (Biel et al., 2009) and nicotine has been shown to decrease Ih current in cortical neurons (Griguoli et al., 2010). We therefore examined developmental- and nicotine-induced changes in the Ih current of both cholinergic and non-cholinergic LDT neurons.
A significantly smaller Ih current amplitude was present in LDT cells from group I (n= 80) when compared to the amplitude of this current in cells from group II (n= 91) (Fig 3 A). The amplitude of Ih current in group II was 30.3 % and 33.3 % larger than that of group I at hyperpolarizations to −100 mV and −110 mV respectively (two-tailed t-test p< 0.05; Fig 3 B). In cells identified as bnos positive, the Ih current amplitude in group II (n= 59) was significantly larger when compared to bnos positive cells from group I (n= 52) (−100 mV: 30.1 % larger; −110 mV: 41.4 % larger; two-tailed t-test; Fig 3 C). There was no significant difference in the Ih current amplitude of bnos negative cells between group I and group II (group I: −100 mV: 13 ± 4 pA; −110 mV: 19.9 ± 5.4 pA, n= 5; group II: −100 mV: 21 ± 3 pA; −110 mV: 27.9 ± 4.5 pA, n= 8; two-tailed t-test).
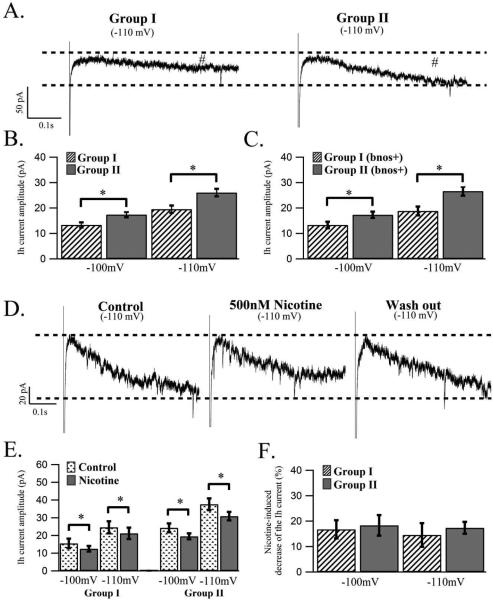
Figure 3.
Developmental- and nicotine-induced effects on the amplitude of the Ih current present in LDT neurons. (A) Whole-cell, voltage clamp recordings showing the Ih current (apparent at #) elicited by a hyperpolarization to −110 mV for 500 ms in a cholinergic LDT neuron from group I (left panel) and group II (right panel) indicating a larger Ih current from the group II cell when compared to the Ih current from the group I cell. (B) Histogram showing the mean Ih current amplitude for LDT neurons in response to hyperpolarizations to −100 mV and −110 mV for a population of cells in group I and group II. Significantly larger Ih current amplitudes were found in group II as compared to group I for both hyperpolarization steps. (C) The larger Ih current amplitude of group II cells compared to group I was confirmed in a population of identified cholinergic LDT neurons for both hyperpolarizations to −100 mV and −110 mV as shown in the histogram. (D) Nicotine decreased the Ih current, which makes a partial recovery following washout as shown by this representative voltage clamp recording of a cholinergic LDT neuron showing the Ih current elicited by a hyperpolarization to −110 mV for 500 ms before, in the presence of 500 nM nicotine and following a 10 minute washout period. (E) The mean Ih current amplitudes from the population of group I and group II LDT neurons in response to hyperpolarizations to −100 mV and −110 mV before and in the presence of 500nM nicotine are shown in a histogram. Although nicotine significantly decreased the Ih current amplitudes for both hyperpolarization steps across both age groups there was no significant difference between the two age groups in this reduction. (F) Histogram showing the percentage decreases of the Ih current amplitude by 500 nM nicotine for hyperpolarizations to −100 mV and −110 mV in group I and group II indicating that nicotine decreases the Ih current amplitude similarly in the two age groups examined.
After clarification that the amplitude of current carried by this channel varied across age with a larger Ih current present in cholinergic neurons from group II animals, we examined the effects of nicotine on this current. Nicotine (500 nM, bath application) significantly decreased the amplitude of Ih current in LDT neurons in both age groups (group I: −100 mV: 16.8 ± 3.6 % decrease; −110 mV: 14.6 ± 4.6 % decrease; n= 11; group II: −100 mV: 18.4 ± 4.1 % decrease; −110 mV: 17.4 ± 2.4 % decrease; n= 8; two-way repeat measurements ANOVA; Fig 3 D and E). However, there was no significant difference in the nicotine-induced decrease of the Ih current when compared across the two age groups (three-way ANOVA; Fig 3 F). In cells identified as bnos positive, nicotine significantly decreased the Ih current amplitude in both age groups (group I: −100 mV: 15.2 ± 5.8 % decrease; −110 mV: 18.1 ± 4.4 % decrease; n= 5; group II: −100 mV: 15.5 ± 4.8 % decrease; −110 mV: 14.5 ± 2.9 % decrease; n= 5; two-way repeat measurements ANOVA), but there was likewise no difference in the nicotine-induced decrease of the Ih current across age for bnos positive cells (three way ANOVA). Across age it was possible to record a partial recovery of the Ih current in 7 cells following a washout period of 10 min (−110 mV: 72 ± 20.9 % recovery; Fig 3 D), which indicates that the nicotine-induced decrease of the Ih current did not stem from eroding recording conditions.
To summarize, we found a significantly larger Ih current amplitude in cholinergic LDT neurons from P16-P34 animals when compared to that elicited in LDT neurons from P7-P15 animals. This suggests the possibility of an increase in Ih channel number or alterations in composition of subunits, which could confer differential sensitivity to intracellular effectors in LDT neurons across ontogeny. While nicotine decreased the Ih current amplitude in cholinergic LDT neurons in both age groups, the nicotine-induced decrease was similar across the two age groups. These data suggest that one effect of nicotine is to reduce a voltage-sensitive current in cholinergic LDT neurons which opposes changes in membrane voltage. As contributions of this current to membrane excitability are presumably greater in animals from the older age group, nicotine's action of reducing this current would be expected to have a larger impact on firing properties in LDT neurons from animals pertaining to the older age group.
3.05 Nicotine induces a larger increase in the frequency of spontaneous excitatory postsynaptic currents (sEPSCs) in P7-P15 as compared to P16-P34 animals
Synaptic input directed to LDT neurons shapes the excitability of the postsynaptic cell. We have shown that nAChRs located on glutamatergic and GABAergic neurons presynaptic to cholinergic and non-cholinergic LDT cells can be functionally activated by nicotinic agonists (Ishibashi et al., 2009). Therefore, we wished to examine whether nicotinic activation of these presynaptic inputs changes across ontogeny. However, there have been no reports of age-related changes in synaptic input within the LDT. Accordingly, we examined the frequency of endogenous sEPSCs, measured as interval between events, across age. As pruning of glutamatergic synapses occurs with age, we anticipated a higher frequency of excitatory synaptic events in neurons from younger animals. However, contrary to our expectations, the interval between sEPSCs in cells from group I (n= 73) was 199 % greater when compared to that of cells from group II (n= 53) (two-tailed t-test p< 0.05; Fig 4 A and B), indicating a higher frequency of excitatory synaptic input directed to postsynaptic cells of older animals. Furthermore, this finding was confirmed in cells identified as bnos positive, where a significantly greater interval between sEPSCs was seen in group I (n= 22) when compared to intervals measured between excitatory events from group II (n= 24) (295.9 % longer interval in group I; two-tailed t-test; Fig 4 C). In bnos negative cells, we found a 98 % longer interval between sEPSCs in group I when compared to the interval between sEPSCs in group II, however, the difference was driven by two cells and found across the population of cells to be non-significant (group I: 1168.1 ± 289.7ms interval, n= 9; group II: 590 ± 179.5ms interval, n= 14; two-tailed t-test). When we examined the amplitude of the endogenous sEPSCs we found no difference in this parameter between the two age groups for the entire cell population (group I: 33.17 ± 0.9 pA; group II: 33.84 ± 1.14 pA; two-tailed t-test p> 0.05), nor was there a difference in the amplitude of the sEPSCs between group I and group II for cells identified as bnos positive (group I: 35.8 ± 4 pA; group II: 29.7 ± 1 pA; two-tailed t-test p> 0.05). Taken together, these data suggest that there is an enhancement in endogenously-triggered release from glutamatergic presynaptic inputs directed to cholinergic LDT neurons across ontogeny.
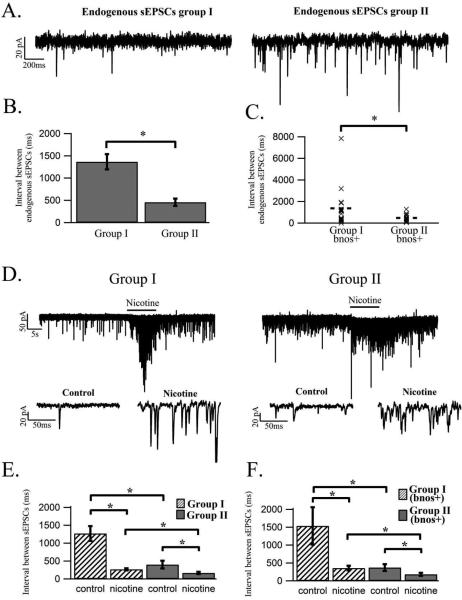
Figure 4.
Nicotine induces a larger increase in sEPSC frequency in P7-P15 animals while the endogenous frequency of sEPSCs increases across ontogeny. (A) The endogenous frequency of sEPSCs, which are shown as downward deflections, was higher in group II when compared to group I as exemplified by these representative whole cell, voltage clamp recordings from a cholinergic LDT neuron from group I (left panel) and group II (right panel). (B) Histogram showing the endogenous interval between sEPSCs in a population of cells from group I and group II. These data indicate a significantly longer interval between individual sEPSCs in group I as compared to the interval in group II. (C) Scatter plot of the endogenous inter sEPSC interval in bnos positive LDT neurons in group I and group II, demonstrating that the interval between sEPSCs is significantly larger in group I compared to the interval between events in group II in cholinergic LDT cells. The dotted line in each column indicates mean values. (D) The left panel is a representative whole cell voltage clamp recording in which the changes in frequency and amplitude of sEPSCs in a cholinergic LDT neuron in response to a puff application of nicotine (10 M, 10s) can clearly be seen. In the right panel the nicotine-induced response in a cholinergic LDT cell from group II is shown. In both age groups representative insets from the control and nicotine portion of the recordings are shown at high gain underneath the recordings at the lower gain. (E) Histogram of the interval between sEPSCs for a population of cells from group I and group II in response to nicotine is shown indicating a significant decrease in the interval between sEPSCs for both groups. Although a lower nicotine-induced interval between sEPSCs was found in group II the percentage decrease of the interval is larger in cells from group I, which also extended to cholinergic cells. (F) The nicotine-mediated decrease in the interval between sEPSCs for cholinergic cells is shown in a histogram where a significant decrease in this parameter is seen in both age groups.
After having established that different frequencies of endogenous sEPSCs were present across ontogeny, we next examined whether differential actions of nicotine (10 μM puff application, 10s) on sEPSCs occurred across age. We found that significantly more cells in group I as compared to group II responded to nicotine with an increase in sEPSC frequency (KS-test p< 0.05) (group I: 67.6 %, n= 74; group II: 49.1 %, n= 53; χ2-test). Furthermore, the nicotine-induced decrease in the sEPSC interval was significantly larger in responding cells within group I, when compared to the decrease in sEPSC interval of responsive cells in group II (69.3 ± 2.4 % decrease in group I vs 48.1 ± 4 % decrease in group II; two-tailed t-test; Fig 4 D and E). The age-associated difference in nicotine-induced increases in frequency of sEPSCs was present in bnos positive cells (group I 64.6 ± 5 % decrease in interval, n= 14; group II 44.5 ± 5 % decrease in interval, n= 14; two-tailed t-test p< 0.05; Fig 4 F); whereas, no significant difference was seen in bnos negative cells (group I n= 5; group II n= 4; two-tailed t-test).
While age-related differences did exist in the numbers of cells exhibiting an increase in frequency of sEPSCs and the frequency increase was greater in cells from the younger age group, there were no differences in the amplitude of nicotine-induced sEPSCs across age. The number of cells with a nicotine-induced increase in the amplitude of sEPSCs (KS-test p< 0.05) did not differ between group I and group II (group I: 41.1 %, n= 73; group II: 37.3 %, n= 53; χ2-test p> 0.05) and there was no difference in the amplitude of nicotine-induced sEPSCs in cells identified as bnos positive between the two age groups (group I: 16.4 ± 2.5 % increase, n= 6; group II: 13.7 ± 2,5 % increase, n= 10; two-tailed t-test p> 0.05). Taken together, our data suggest that nicotine results in a larger enhancement of glutamatergic release onto postsynaptic cholinergic neurons in animals from the younger age group than that elicited in animals from the older age group.
To examine differential decreases of nicotine-induced sEPSCs following repeat nicotine exposures, a second nicotine application was applied five minutes after the initial application. We, however, found no difference in the number of cells responding to nicotine with an increase in frequency across both nicotine applications between group I and group II (group I: 42 %, n= 50; group II: 38.5 %, n= 26; χ2-test p> 0.05). In responding cells the higher frequency of nicotine-induced sEPSCs in group I animals observed in the first application also extended to effects elicited by the second application (group I: 58.9 ± 3.2 % decrease in interval, n= 21; group II: 41.9 ± 4.8 % % decrease in interval, n= 10; two-tailed t-test p< 0.05). We did not find any significant differences in the attenuated response to a second nicotine application for either the nicotine-induced increase in frequency or amplitude across the two age groups. We conclude that while there were no differences in the attenuation of sEPSC frequency and amplitude between the first and second application of nicotine across the two age groups, nicotine induced a larger increase in frequency of sEPSCs in animals from the younger age group when compared to animals from the older age group and this extended to repeat nicotine applications.
3.06 Animals from the older age group have a higher frequency of miniature EPSCs (mEPSCs) in LDT neurons
We have shown in conditions of synaptic blockade that nAChRs located on presynaptic terminals impinging on LDT neurons can cause nicotine-mediated glutamate release onto the cholinergic postsynaptic cell (Ishibashi et al., 2009). Accordingly, age-related nicotine-induced increases in sEPSCs could be due to nAChRs located on terminals directed to postsynaptic cholinergic LDT cells, however, they could also be due to alterations in nAChRs located at more distal sites. We wished to determine whether nAChRs located on terminals can be differentially activated across age and therefore recorded EPSCs in the presence of blockade of action potentials by inclusion of TTX in the ACSF (mEPSCs). However, initial investigations were directed at confirming whether ontogenetic differences in endogenous sEPSCs extended to mEPSCs.
In LDT neurons from group II, there was a significantly greater frequency of endogenous mEPSCs as compared to group I (group I: interval 675.9 ± 125.4 ms, n= 26; group II: interval 369.4 ± 48.3 ms, n= 25; two-tailed t-test). There was no difference in the amplitude of endogenous mEPSCs between the two groups (group I: 15.2 ± 0.7 pA; group II: 16.1 ±1 pA; two-tailed t-test p> 0.05). This supports the conclusion that ontogenetic differences in glutamatergic terminals exist which lead to increased release of excitatory neurotransmitter in older animals.
We did not find any difference in the number of cells responding to nicotine (10 M puff application, 10s) with an increase in mEPSC frequency (KS-test p<0.05; Fig 5 A) between the two age groups (group I: 92.3 %, n= 26; group II: 76 %, n= 25; χ2-test), which extends to identified cholinergic neurons (group I: 100 %, n= 9; group II: 82.4 %, n= 17; χ2-test p> 0.05). Nor was there a significant difference in the nicotine-induced increase in mEPSC frequency or amplitude across the two groups. These results suggest that the larger nicotine-induced frequency of sEPSCs found in group I as compared to group II is mediated, not by differences in nAChR situated on presynaptic glutamatergic terminals but by nAChRs more distally located.
Figure 5.
Nicotine elicits increases in frequency and amplitude of mEPSC and mIPSC and induces differential increases in mIPSC amplitude across ontogeny. (A) Representative whole cell, voltage clamp recordings showing first and second nicotine (black line) mediated elicitations of mEPSCs and mIPSCs from a cholinergic LDT neuron from group I (A1 and A2) and group II (A3 and A4). In the bottom of each recording a representative inset of a part of the recording during nicotine exposure is shown. The first nicotine application produced mEPSCs and mIPSCs in both cells shown, but a second application of nicotine in the same cells elicited a smaller proportion of mIPSCs as compared to mEPSCs in both age groups. (B) The percentage of cells responding to two nicotine applications with an increase in mEPSCs and mIPSCs across both applications is displayed in a histogram where a significantly larger proportion of cells show the appearance of mEPSCs as compared to mIPSCs suggesting that a longer recovery period is required for nicotine to induce repeat mIPSCs compared to mEPSCs. (C) Histogram showing the endogenous mIPSC amplitude in group I and group II indicating larger amplitude events occur in group II than in group I. (D) Nicotine induced significantly larger mIPSC amplitudes in cells from group II as compared to the amplitude induced in group I as illustrated by the cumulative fractions distribution of the amplitude of all nicotine-induced mIPSCs in group I (grey) and group II (black) for LDT cells responding to nicotine with a significant increase in amplitude. (E) The larger nicotine-mediated mIPSCs amplitude in group II as compared to group I extended to bnos positive LDT cells as indicated by the cumulative fractions distribution showing the amplitude of nicotine-induced mIPSCs from bnos positive cells from group I and group II.
3.07 Nicotine induces larger amplitudes of miniature inhibitory events (mIPSCs) in animals from the younger age group
Since we established that fewer mEPSCs were present in neurons from P7-P15 animals, we also examined whether differences in endogenous inhibitory synaptic input existed across age. However, despite the increase in the chloride driving force in recorded cells conferred by the low chloride concentration in our patch solution, we only observed sIPSCs in a minority of cells. However, by reducing the membrane potential to −40 mV, thereby increasing the electrical potential for the chloride driving force, in the presence of TTX to block depolarization-induced action potentials, mIPSCs were visible in all cells. The endogenous frequency of mIPSCs was not significantly different between group I and group II (group I: 2488.8 ± 329.1ms interval, n= 26; group II: 1999.7 ± 248.6ms interval, n= 25; two-tailed t-test). We did, however, find a significantly larger amplitude of endogenous mIPSC in group II as compared to group I (group I: amplitude = 11 ± 0.5 pA; group II: amplitude = 14.2 ± 0.6 pA; two-tailed t-test; Fig 5 C) and this phenomenon extended to bnos positive cells (group I: amplitude = 11.4 ± 0.8 pA, n= 9; group II: amplitude = 13.6 ± 0.6 pA, n= 17; one-tailed t-test p< 0.05). These data which indicate age-related differences in amplitude, but not frequency, of mIPSCs suggest that differences in the number and/or properties of postsynaptic inhibitory receptors, likely GABAergic (Ishibashi et al., 2009), occur across ontogeny in cholinergic LDT neurons.
Nicotine-mediated inhibitory input to postsynaptic cholinergic cells, which will reduce the excitability of the cell, have been shown to arise from nAChRs located on presynaptic GABAergic terminals (Ishibashi et al., 2009). Age-related changes in these nAChRs could subsequently induce differential inhibitory input to postsynaptic LDT neurons. Therefore, we examined the nicotine-induced increase in mIPSC frequency and amplitude across the two age groups but found no difference in either the frequency (group I: 84 ± 4.2 % decrease in interval, n= 13; group II: 84.5 ± 3.4 % decrease in interval, n= 13: two-tailed t-test p> 0.05) or the amplitude of nicotine-induced inhibitory events (group I: 56.2 ± 6.5 % increase, n= 15; group I: 44.7 ± 5.1 % increase, n= 13; two-tailed t-test p> 0.05) of responsive cells (KS-test p<0.05) between the two age groups. While there was no difference in the percentage increase between the two age groups, nicotine did induce larger amplitude events in responsive cells from group II when compared to those elicited in cells from group I, which extended to bnos positive cells (KS-test p< 0.05; Fig 5 D and E). To quantify this action we examined the number of cells exhibiting nicotine-induced mIPSC amplitudes exceeding 40 pA, which is approximately three times the amplitude of endogenous mIPSCs in group I cells, and found a significantly larger proportion of cells in group II as compared to group I exhibiting events exceeding this value (group I: 33.3 %, n= 15; group II: 76.9%, n= 13; χ2-test). In bnos positive cells a larger proportion of cells showing mIPSC events above 40 pA was seen in group II as compared to group I (group I: 16.7 %, n= 6; group II: 77.8 %, n= 9; χ2-test p< 0.05). These data indicate that nicotine induces larger amplitude inhibitory synaptic events in cholinergic LDT cells from animals belonging to the older age group, indicating that exposure to nicotine would induce a larger relative degree of postsynaptic inhibitory drive in this population.
3.08 Recovery of effect takes longer for nAChRs on presynaptic GABA cells vs presynaptic glutamate cells
Differences in desensitization kinetics of nAChRs located on GABAergic and glutamatergic presynaptic inputs directed to DA-containing VTA cells leading to a longer silencing of GABAergic cells is believed to underlie a long-lived excitatory response to nicotine within the VTA ((Mansvelder et al., 2002); however see (Tolu et al., 2013)). Differential desensitization properties between nAChRs mediating excitatory and inhibitory synaptic input across the two age groups could exist in the LDT and thereby significantly alter cellular responses during persistent nicotine exposure. Accordingly, we examined possible differential responsiveness of excitatory and inhibitory presynaptic inputs to a repeat application of nicotine.
While there was a significant decrease in the frequency of mEPSCs and mIPSCs elicited by a second application of nicotine as compared to the initial nicotine application in both age groups, there was no difference across age in the number of cells showing increases in either mEPSC or mIPSC frequency, nor was there a difference in the decrease in frequency between mEPSC or mIPSC upon repeat nicotine application across age. Therefore, age groups were pooled for further studies. We next examined the degree of nicotine-induced repeat effects on inhibitory events compared to excitatory events across the entire cell population and found a significantly smaller number of cells showing an increase in the frequency of mIPSCs as compared to those showing increases in mEPSCs with repeat nicotine applications (mEPSCs: 86.7 %, n= 30; mIPSCs: 52.4 %, n= 21; χ2-test; Fig 5 A and B). In bnos positive cells a similar phenomenon was seen (mEPSCs: 80 %, n= 15; mIPSCs: 38.5 %, n= 13; χ2-test p< 0.05).
Our data indicate that, while no differences existed in the repeatability of mEPSCs and mIPSCs across the two age groups examined, nAChRs located on GABA-containing presynaptic terminals show a reduced responsiveness to repeat nicotine applications as compared to nAChRs located on glutamate-containing terminals which corresponds to previous findings in the VTA (Mansvelder et al., 2002). Accordingly, upon persistent nicotine exposure excitatory synaptic input directed to postsynaptic cholinergic LDT neurons would be favored.
3.09 Developmental- and nicotine-induced changes of the action potential spike shape and afterhyperpolarization (AHP)
Our data suggest that nicotine induces larger inward currents, rises in calcium and sEPSCs in LDT cells from animals belonging to the younger age group examined, which suggest that LDT neurons in P7-P15 animals might be more excited by nicotine than those from P16-P34 animals. Nicotine could further excite LDT cells by affecting parameters involved in action potential formation, threshold and afterhyperpolarization which has been shown to occur in other cell types (Good et al., 2006; Kawai et al., 2007; Liu et al., 2004; Ma et al., 2013; Wang et al., 1999; Wang et al., 2000a) However, before exploring the effect of nicotine on these parameters, we examined the occurrence of any ontogenetic alterations.
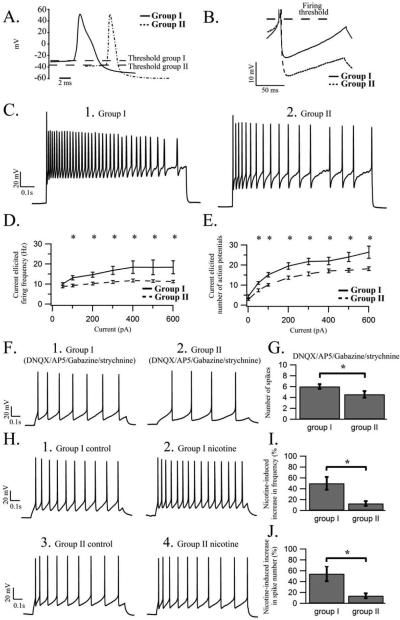
In preliminary studies, we noted that injection of a significantly lower current was required for the induction of single action potentials in cells from the younger as compared to the older age group, which could be due to a more hyperpolarized action potential firing threshold. However, contrary to our expectations, a more depolarized firing threshold was exhibited by cells from group I, when compared to that exhibited by cells from group II (see table I; Fig 6 A), and this extended to a subpopulations of cells identified as bnos positive. The spike width1/2 max was significantly broader in group I when compared to that of group II for the entire population of cells as well as in a subset of recorded cells identified as bnos positive. The spike amplitude also changed across ontogeny as we observed a larger spike amplitude in group II when compared to the spike amplitude of cells from group I. Greater spike amplitudes were also seen in a population of bnos positive cells from group II when compared to a population of bnos positive cells in the younger age group. Both the maximum rise and decay slopes of the action potential were significantly smaller in group I when compared to values for these same parameters in cells from group II, which also extended to a subpopulation of bnos positive cells (see table I; Fig 6 A). The findings of developmentally-related changes in spike width1/2 max, amplitude as well as the rise and decay slope corroborates results detected in an earlier study conducted in C57BL6 mice (Kristensen et al., 2007).
Table 1.
Christensen et al., 2014
| Threshold (mV) | Spike width1/2 max (ms) | Spike amplitude (mV) | Rise slope Max (mV/ms) | Decay slope max (mV/ms) | AHP amplitude (mV) | AHP minimum (mV) | |
|---|---|---|---|---|---|---|---|
| All cells | |||||||
| Group I (n= 57) | −30.4 ± 0.57 | 1.62 ± 0.05 | 80.1 ± 1.13 | 170 ± 9 | −46.4 ± 2 | −17.6 ± 0.59 | −48.1 ± 0.64 |
| Group II (n= 75) | −34.1 ± 0.53* | 1.23 ± 0.02* | 84.8 ± 0.84* | 220.5 ± 8* | −63.4± 2* | −20.1 ±0.4* | −54.2 ± 0.51* |
| Bnos positive cells | |||||||
| Group I (n= 40) bnos+ | −30.8 ± 0.67 | 1.67 ± 0.05 | 81.4 ± 1.25 | 173.3 ± 11 | −44.9 ± 2 | −17.7 ± 0.61 | −48.4 ± 0.73 |
| Group II (n= 50) bnos+ | −34.6 ± 0.64* | 1.26 ± 0.03* | 84.8 ± 0.95* | 215 ± 10* | −61.3 ± 2* | −20.3 ± 0.61* | −54.9 ± 0.59* |
Developmental-induced alterations in parameters governing the shape of the action potential and AHP. In the top part of the table, measured values for the examined parameters of the action potential across group I and group II are shown. The bottom half of the table shows the values of the examined parameters for bnos positive cells from group I and group II.
denotes statistical significances using a two-tailed t-test between the two age groups.
Figure 6.
Nicotine application induces alterations in the action potential spike shape, AHP and firing rate of LDT neurons distinct from changes in there features occurring across ontogeny. (A) The spike shape of an evoked action potential from a cholinergic group I (black) and group II (dotted) LDT cell is shown and as can clearly be seen the action potential firing threshold (dotted line) for the group I cell is more depolarized than the threshold for the group II neuron. (B) High gain image of the AHP of a cholinergic group I (black) and group II (dotted) LDT cell, overlaid based on their firing threshold (dotted line), showing a larger and more hyperpolarized AHP from the group II as compared to the group I cell. (C) Representative whole-cell current clamp recording of a cholinergic LDT neuron from a group I (C1) and group II cell (C2) are shown in which a 1000ms current pulse of sufficient size to elicit the maximum number of action potentials was applied. The recording from the group I cell displays a larger number of elicited action potentials compared to the recording from the group II cell. (D) The average action potential firing frequency for current pulses of increasing current strengths for group I (black line, n= 11) and group II cells (dotted line, n= 16) are shown in a graph indicating that across the population of cells a significantly higher action potential firing frequency is present in group I when compared to that of group II. At current pulses above 600pA spike failure was induced in most cells from group I and comparison across groups was therefore no longer possible. (E) Graph showing the mean number of elicited spikes with increasing current pulses for group I (black line) and group II (dotted line) cells with a significantly larger number of elicited action potentials present in group I as compared to group II. (F) Representative current clamp recording displaying the number of action potentials elicited by a 10 pA current pulse in the presence of DNQX, AP5, GZ and strychnine in cholinergic LDT neurons from a group I (F1) and group II (F2) cell indicating that a larger number of elicited action potentials are seen in group I as compared to group II cells even with blockade of excitatory and inhibitory amino acids. (G) Histogram showing the average number of action potentials elicited by a 10 pA current pulse in the presence of DNQX, AP5, GZ and strychnine in the population of group I and group II cells. The number of action potentials elicited in group I is significantly greater when compared to that of group II. (H) Whole-cell current clamp recordings of a (H1 and H2) group I and (H3 and H4) group II LDT cell showing action potential trains elicited by the same current pulse before and in the presence of 200μl bath applied nicotine (10mM) when the holding current has been corrected to negate nicotine-induced changes in the membrane potential. The recordings suggest that in the presence of nicotine a larger increase in the number of elicited spikes occur in group I as compared to group II cells. (I) Histogram showing, for group I and group II, the percentage increases in the spike frequency elicited by a fixed current pulse in the presence of nicotine compared to control conditions. The frequency of the action potentials elicited in the presence of nicotine when compared to the frequency in control conditions is significantly larger in group I as compared to group II. (J) The percentage increase in the number of spikes elicited by a fixed current pulse in the presence of nicotine compared to control for group I and group II are shown in a histogram and indicate that a significantly larger increase in the number of action potentials elicited in the presence of nicotine is present in group I as compared to group II.
We found that the amplitude of the AHP in LDT neurons from group II was significantly larger when compared to the AHP amplitude of cells from group I. Cells identified as bnos positive showed a similar phenomenon. The AHP minimum was also significantly more hyperpolarized in neurons from group II as compared to group I in both the entire cell population and in cells identified as bnos positive (see table I; Fig 6 B). These results demonstrate that across ontogeny, multiple parameters of the spike shape and AHP alter, which could differentially influence cellular excitability. The more hyperpolarized firing threshold and narrower spike width seen in group II would be expected to lead to a heightened frequency of firing in LDT neurons in animals from the older age group, while the smaller amplitude of the AHP exhibited by neurons in group I could decrease the action potential recovery rate and thereby facilitate firing frequency in cells of animals belonging to the younger age group. The changes seen in the rise and decay slope of the action potential spike across ontogeny were unsurprising and indicate that in cholinergic LDT neurons, similar to other brain regions (Baumgold et al., 1983; Couraud et al., 1986; Huguenard et al., 1988; McCormick and Prince, 1987), developmental changes in Na+ and K+ channel density affecting the action potential rise and decay phase are occurring.
Following establishment of developmental changes in the profile of the action potential and the AHP, we preceded to examine nicotine-induced (500 nM, bath application) changes on these parameters when the cell was held at a fixed membrane potential (−60 mV). Across the different developmental ages, we found a significantly larger nicotine-induced increase in the spike width1/2 max in cells from group II when compared to nicotine induced increases in cells from group I (group I: 0.3 ± 2 % increase, n= 11; group II: 11.2 ± 2.9 % increase, n= 5; two-tailed t-test). An insufficient number of bnos positive cells were recovered and identified to determine whether the phenomenon was also present in cholinergic cells. We found no difference in the nicotine-induced response across the developmental ages examined in the remaining parameters; therefore, data have been pooled for reporting of nicotinic alterations of these properties (table II). Nicotine did not significantly affect the firing threshold contrary to previous findings (Kawai et al., 2007). Nicotine significantly decreased the spike amplitude (5.1 ± 1.8 % reduction, n= 16,), a phenomenon also seen in bnos positive cells (n= 8) (see table II). As nicotine activates nAChRs permeable to Na+ and K+ and has actions on other membrane conductances (Griguoli et al., 2010; Nutter and Adams, 1995), nicotine-mediated reductions in spike amplitude could be due to shunting of current across the membrane due to alterations in membrane conductance (Zhang et al., 2004). In addition, we also observed a 8.8 ± 3.8 % decrease of the maximum decay slopes of the action potential in the presence of nicotine, which was significant and a similar phenomenon was seen in bnos positive cells (8.7 ± 4.9 %, p< 0.05). Nicotine did not significantly affect the maximum slope of the rise of the action potential in the population of recorded cells, which included a subpopulation of cells identified as bnos positive. In the presence of nicotine, the AHP minimum was, however, significantly less hyperpolarized as compared to control conditions (2.2 ± 0.8 %). Based on these results, we conclude that nicotine affects the profile of the action potential and by decreasing the AHP minimum, nicotine may decrease the AHP recovery period and thereby increase cellular excitability within cells from the range of ages studied. This action may be more functionally relevant in enhancing firing in neurons from older animals, which demonstrate a lower AHP minimum than that present in neurons from younger animals. The nicotine-induced increase of the spike width in LDT neurons from older animals would be expected to reduce the firing rate to a greater degree than reductions in firing rate induced by this nicotine mediated change in cells from younger animals.
Table 2.
Christensen et al., 2014
| Threshold (mV) | Spike width1/2 max (ms) | Spike amplitude (mV) | Rise slope max (mV/ms) | Decay slope max (mV/ms) | AHP amplitude (mV) | AHP minimum (mV) | |
|---|---|---|---|---|---|---|---|
| All cells | |||||||
| Control (n= 16) | −32.3 ± 1.1 | 1.58 ± 0.1 | 79,03 ± 2,93 | 186.6 ± 14 | −48.3 ± 4 | −20.19 ± 1 | −52.52 ± 0.8 |
| Nicotine (n= 16) | −31.5 ± 1.1 | 1.63 ± 0.1 | 74,66 ± 2,66* | 166.4 ± 11 | −42.9 ± 4* | −19.94 ± 1.1 | −51.44± * |
| Bnos positive cells | |||||||
| Control (n= 8) bnos+ | −33.8 ± 1.5 | 1.66 ± 0.1 | 82.48 ± 3.89 | 187.1 ± 20 | −46.6 ± 5 | −19.25 ± 1.3 | −53 ± 1.2 |
| Nicotine (n= 8) bnos+ | −32.7 ± 1.4 | 1.73± 0.1 | 77.54 ± 3.96 | 174 ± 19 | −42.3 ± 5* | −19.44 ± 1.6 | −52.1 ± 1.6 |
Nicotine changes the action potential shape and AHP. The measured values for the examined parameters of the action potential before and in the presence of 500nM bath applied nicotine are shown for both the entire population and the bnos positive population.
denotes statistical significances using a two-tailed paired t-test across conditions and suggest that nicotine affects some of the currents involved in shaping action potentials.
3.10 Nicotine induces a larger increase in cellular excitability in LDT neurons in animals from the younger as compared to the older age group
As we have previously shown that nicotine enhances the firing rate of cholinergic LDT neurons (Ishibashi et al., 2009), it was of functional interest to examine whether age-related differences in this action were present. Accordingly, we compared the firing frequency and numbers of action potentials elicited between the two age groups.
Neurons from group I (n= 11) exhibited an higher average steady state firing frequency between a range of current applications when compared to the firing rate of cells from group II (n= 16) (two-way repeated measurements ANOVA, p< 0.05; Fig 6 C and D). In accordance with this finding, a significantly larger number of action potentials elicited by current pulses were seen in group I when compared to the number of action potentials elicited in this same range of current strengths from cells in group II (two-way repeated measurements ANOVA; Fig 6 E). In bnos positive cells, a higher firing frequency and a larger number of action potentials were seen in cells from group I (n= 8) as compared to values found for these measures in cells from group II (n= 8) (two-way repeated measurements ANOVA p< 0.05). Additionally, the current strength required to elicit the maximum number of action potentials was significantly lower in cells from group I when compared to the current required to elicit the maximal number of action potentials in neurons from group II (group I: 446.5 ± 72 pA; group II: 694.4 ± 60 pA; two-tailed t-test), and a similar phenomenon was seen in cells identified as bnos positive (group I: 538.5 ± 63.8 pA; group II: 775.3 ± 62.7 pA; two-tailed t-test p< 0.05). To ensure that differences in membrane depolarization did not underlie the difference in firing frequency, we examined the firing frequency in a subset of cells from both age groups held at the same potential (−40 mV). In cells from group I, a higher firing frequency was elicited when compared to the firing frequency induced in group II (group I: 13.5 ± 1.8 Hz, n= 5; group II 10 ± 0.3 Hz, n= 7; two-tailed t-test p< 0.05).
As we have shown that excitatory glutamatergic and to a limited extent, GABAergic, input remains in the slice, which could contribute to differential firing rates across age, we also examined the number of elicited action potentials in response to a 10 pA current pulse in the presence of blockade of glutamatergic and GABAergic receptors located on the postsynaptic cells by inclusion of DNQX, AP5, Gabazine and strychnine in the ACSF. A significantly larger number of action potentials were elicited at a fixed current strength from neurons of group I (n= 28) when compared to the numbers elicited from group II (n= 18) (one-tailed t-test; Fig 6 F and G). These data indicate that the enhanced firing frequency seen in neurons from the younger age group involves ontogenetic differences in postsynaptic mechanisms not involving glutamate, glycine or GABA receptors.
When current was applied to maintain the resting membrane potential so as to negate the contribution of voltage-activated conductances, nicotine application (200 l, 10 mM, pipette applied) resulted in a larger increase in steady state firing frequency in cells from group I when compared to the increase in firing elicited in group II neurons (group I: control 8.8 ± 0.3 Hz; nicotine 13.3 ± 1.2 Hz, n= 7; group II: control 8.1 ± 0.2 Hz; nicotine 9.1 ± 0.3 Hz, n= 10; two-tailed t-test p< 0.05; Fig 6 H and I). Additionally, the presence of nicotine resulted in a significantly larger increase in the number of elicited action potentials in neurons from group I, when compared to numbers elicited in cells from group II (group I: control 10.4 ± 0.4; nicotine 16 ± 1.4; group II: control 9.5 ± 0.3; nicotine 10.8 ± 0.5; two-tailed t-test; Fig 6 J). In cells identified as bnos positive, a larger number of elicited action potentials (group I: control 10.8 ± 0.4; nicotine 14.25 ± 0.6 (n=4); group II: control 9.8 ± 0.4; nicotine 10.4 ± 0.7 (n=5); one-tailed t-test p< 0.05) as well as a larger steady state firing frequency (group I: control 9 ± 0.3 Hz; nicotine 11.6 ± 0.7 Hz; group II: control 8.4 ± 0.3 Hz; nicotine 8.9 ± 0.5 Hz; one-tailed t-test p< 0.05; Fig H) was also found to be elicited by nicotine in cells from group I when compared to changes elicited by nicotine in these measures in cells of group II. To rule out differences in contribution of input resistance across cells we examined the nicotine-induced firing frequency in five cells across age in which the final depolarization was the same for each cells. Under these conditions in which variations in input resistance are negated, the nicotine-induced increase in firing frequency in cells from group I was larger than the increase induced by nicotine in group II (group I: 56.8 ± 0.2 %, n= 2; group II: 3.2 ± 4.6 %, n= 3; two-tailed t-test p< 0.05). Taken together, these data indicate that cholinergic LDT neurons exhibit a higher firing frequency in animals from the younger age group than that exhibited in animals pertaining to the older age group and nicotine enhances this frequency to a larger extent in younger animals when compared to nicotine-induced enhancements in cells from older animals. These data indicate that nicotine exposure would result in differences in firing in cholinergic cells across ontogeny, possibly leading in younger animals to a larger cholinergic output from LDT neurons to target regions, including those within the VTA.
4. Discussion
This study was designed to elucidate whether nicotine, which is associated with an age-related risk of developing dependency, has differential actions across ontogeny on cholinergic LDT neurons, as these cells are importantly involved in addiction-related physiology. Our data indicate that nicotine exerts greater excitatory effects on cholinergic LDT neurons in P7-P15 animals when compared to actions in P16-P34 animals. As the LDT plays a role in addiction-related behaviours and processes (Dautan et al., 2014; Forster and Blaha, 2000; Lammel et al., 2012; Lodge and Grace, 2006) the ontogenetic differences in nicotine cellular actions on LDT neurons could be importantly involved in age-associated differences in behavioral actions of this drug.
4.01 Nicotine-induces larger increases in intracellular calcium and larger inward currents in LDT cells from animals belonging to a younger age group
Our initial experiments using ‘bulk-load’ calcium imaging were designed to provide a quick assay to determine whether nicotine elicited differences in neuronal responsiveness across ontogeny. With the caveat in mind that it was not possible with this technique to determine the phenotype of the individual cells examined, calcium imaging experiments revealed that nicotine had differential actions on rises in calcium in unidentified LDT neurons dependent upon age. The larger number of responses and the larger amplitude of calcium rises induced by nicotine in LDT neurons from younger age groups could lead to a differential induction of calcium driven physiology, such as synaptic plasticity and gene expression (Berridge, 1998) between LDT cells from younger as compared to older animals, which could be functional significant. In DA-VTA neurons a single intraperitoneal nicotine injection has been shown to alter glutamatergic neurotransmission by increasing the amplitude of AMPA-mediated EPSCs and induction of this glutamate involved plasticity was reversed by co-administration of the nAChR antagonists methyllycaconitine and mecamylamine (Gao et al., 2010; Saal et al., 2003). Furthermore, a lower nicotine dose was required to increase the AMPA-mediated EPSC amplitude in neurons of younger (P21- P35) compared to older mice (P60-P90), which suggests that DA-VTA neurons from younger mice are more sensitive to the mechanisms underlying this nicotinic-mediated synaptic plasticity (Placzek et al., 2009). While nicotine-induced plasticity has not been shown in the LDT, drug-induced synaptic plasticity has been found to occur in the LDT of cocaine and amphetamine-treated animals (Kurosawa et al., 2013; Nelson et al., 2007), possibly resulting in a larger output to the VTA. Nicotine-mediated synaptic plasticity could therefore occur in the LDT and the large differences in nicotine-induced rises in calcium might suggest a heightened sensitivity to nicotine-mediated synaptic plasticity in animals from younger ages.
Although the mechanisms underlying differences in nicotine-mediated calcium rises were not explored, we did determine in identified LDT neurons, that nicotine elicited current responses in fewer LDT neurons in P16-P34 animals, and in those cells responding, smaller depolarizing currents were induced. As the amplitude of nicotine-induced currents were not altered by the presence of blockers of synaptic activity, our results suggest that reduced nicotine-mediated current amplitudes in neurons from animals pertaining to the older age group likely involving postsynaptic nAChRs could contribute, in part, to smaller calcium rises. Nicotine-induced calcium rises can occur directly through calcium permeable nAChRs or secondarily following depolarization sufficient to activate voltage operated calcium channels. A reduced number of cells responding, and/or smaller amplitude nicotinic-mediated neuronal depolarizations in the LDT in animals from the older age group would be expected to result in reductions of calcium rises via these entry mechanisms.
The developmental decrease in the nicotine-induced inward current could be the result of ontogenetic alterations in the number of nAChRs expressed in the cell membrane. Developmental changes in nAChRs mRNA has been detected in several different brain regions (Azam et al., 2007; Dwyer et al., 2009) and developmental decreases in nicotinic binding sites have been shown in the brain stem (Miao et al., 1998; Zhang et al., 1998). While the point was not specifically addressed in the binding site study, the possibility exists of a developmental decrease in the number of nAChRs within the LDT. However, our Ri study argues against this possibility. Ontogenetic alterations in the specific subunit composition of nAChRs within the LDT could also contribute to the developmental decrease in nicotine-induced current amplitudes, as the specific subunit composition of the individual nAChR determines the affinity for nicotine (Albuquerque et al., 2009). The higher nicotine affinity of, for example, the α4β2-containing nAChR would be expected to produce a greater nicotine response at physiologically relevant nicotine concentrations than that induced by lower affinity nAChRs such as the α7-containing nAChRs. Within the LDT, the presence of mRNA for α7, α4, β4 and β2 nAChR subunits has been noted (Azam et al., 2003), and antagonists exhibiting relative selectivity for α7- and β2-containing nAChRs were found to reduce postsynaptic, inward currents induced by cholinergic agents in LDT neurons in this and other studies (Ishibashi et al., 2009), which indicates the presence of postsynaptic high affinity α4β2-containing nAChRs and low affinity α7-containing nAChRs. Within the developmental period examined, binding of high affinity α4β2-containing nAChR and low affinity α7 by 3H epibatidine and 3H α-bungarotoxin, respectively, drastically decreases in the brain stem suggesting development decreases in both α4β2- and α7-containing nAChRs (Miao et al., 1998; Zhang et al., 1998). Developmental decreases in particular of the high affinity α4β2-containing postsynaptic nAChR as opposed to reductions of other nAChRs subtypes could produce larger decreases in nicotine-mediated inward currents, which could partly underlie the ontogenetic decrease of the nicotine-induced inward current found in the present study in cholinergic LDT cells. The specific stoichiometry of nAChR subunits also influences the affinity of nAChRs for nicotine. The high affinity α4β2-containing nAChR can co-assemble as α4(3)β2(2) or α4(2)β2(3) nAChRs, where the latter has a higher affinity for nicotine when compared to the former (Albuquerque et al., 2009; Nelson et al., 2003). Developmental alterations in nAChR subunit stoichiometry, which favor low affinity nAChR subunit compositions in LDT cells from older animals could therefore contribute to the decrease in inward current found across ontogeny. In addition to different agonist affinities, various nAChR subunit compositions also have differential desensitization kinetics with the high affinity α4β2-containing nAChR showing desensitization at low nM concentrations; whereas the α7-containing nAChR shows initial indications of desensitization at M concentrations (Fenster et al., 1997; Quick and Lester, 2002; Wooltorton et al., 2003). We therefore speculate that the larger decrease in inward current of repeat nicotine applications seen in the younger as compared to the older group could stem from the presence of a larger proportion of α4β2-containing nAChRs in P7-P15 animals whereas a larger proportion of α7-containing nAChRs contribute to the nicotine-induced inward current in the P16-P34 age group. While larger calcium rises in the youngest age group would suggest a higher percentage of the calcium permeable α7-subunit, nicotine induced calcium in LDT cells in this age group could have derived from other subunit-containing, calcium permeable nAChRs, or other sources of calcium. Indeed preliminary results investigating the nAChR subunit composition across ontogeny in LDT cells from mice using nAChR antagonists suggests that in cells from P7-P15 animals the proportion of current induced by cholinergic agents is mediated to a higher degree through α4β2-containing nAChRs, whereas a larger proportion of current in P16-P34 animals is mediated through α7-containing nAChRs. One caveat in our study is that the amplitude of nicotine-induced inward currents may be underestimated as nAChRs undergo desensitization, however, as results suggested that desensitization was greater at P7-P15 when compared to P16-P34 current amplitudes from group I were likely underestimated to a greater extent than the current amplitude from group II which would further exacerbate age-related differences.
4.02 Nicotine decreases the Ih current amplitude
We show for the first time that nicotine decreases the Ih current present in cholinergic LDT neurons, which corroborates findings of a nicotine-mediated decrease of this current seen in cortical neurons (Griguoli et al., 2010). Additionally, our current study confirms developmental changes in Ih current seen in a subset of cholinergic LDT neurons in C57BL6 mice (Kristensen et al., 2007) and extends them to bnos-identified neurons of the LDT in NMRI mice. However, previously we did not observe the presence of Ih current in LDT neurons from animals below 16 days of age. This discrepancy is likely attributable to differences in the experimental procedures, as we previously monitored Ih currents using voltage steps to less negative potentials. Developmental increases in the Ih current have previously been seen in putative cholinergic neurons of the Sprague-Dawley rat pedunculopontine tegmental nucleus (PPT), a cholinergic nucleus located adjacent to the LDT (Good et al., 2006). Although the amplitude of the Ih current did show age-related differences, the nicotine mediated-decrease of the amplitude of the Ih current did not appear to change across ontogeny. These findings suggest that the alteration underlying the age-related differences in the amplitude of Ih currents does not affect the nicotine-mediated attenuation of the Ih current, which has been suggested to be due to direct actions on Ih channels and not via nAChR-mediated mechanisms (Griguoli et al., 2010). While changes in the properties of Ih channels, due to alterations in subunit compositions, have been shown to occur across age (Kanyshkova et al., 2009; Vasilyev and Barish, 2002), changes in subunit compositions would be expected to result in differences in the percentage of attenuation of the Ih current by nicotine across age, as it would be expected that the effects of nicotine on different Ih channel subunits would vary. Changes in the numbers of Ih channels have also been shown to occur across age (Vasilyev and Barish, 2002) and our findings of an equivalent percentage of reduction of this current by nicotine between the two age groups would be consistent with the interpretation that Ih channels show an ontogenetic increase across age. Accordingly, while we have not ruled out developmental changes in Ih channel properties, our data support the conclusion that the increase found in Ih current amplitude across age is mediated by an ontogenetic increase in number of Ih channels, which has also previously been proposed to underlie developmental increases in the size of Ih current in the PPT (Kobayashi et al., 2004). Although the percentage decrease of the Ih current amplitude induced by nicotine did not vary across age, the actual decrease of the Ih current induced by nicotine in cholinergic LDT neurons of animals from the older age group was larger than that of animals belonging to the younger group. The Ih current influences cellular excitability by repolarizing the cell and contributing to the maintenance of the resting membrane potential (Biel et al., 2009), therefore the functional outcome of the nicotinic attenuation of the Ih current could vary across age and possibly play a role in alteration of cellular excitability across ontogeny.
4.03 The frequency of nicotine-induced PSCs changes across ontogeny
Our examination of nicotine-induced increases in sEPSC frequency indicates that nicotine exposure would result in a relatively larger glutamatergic excitation of cholinergic LDT neurons in animals from the younger age group as compared to levels of this excitation stimulated by nicotine in these cells in animals belonging to the older age group. As we did not observe any difference in nicotine-induced mEPSC frequency across age suggesting reliance of this effect on action potential generation, our results indicate that the developmental differences in excitatory inputs to LDT neurons are dependent upon nicotine-activated mechanisms upstream of presynaptic terminals. In addition to fewer nicotine-induced sEPSCs in LDT cells, P16-P34 animals also exhibited an increase in the number of cells showing large amplitude nicotine-induced mIPSCs, which suggests that nicotine mediates a larger inhibitory effect in animals from the older age group possibly through an increase in numbers, or properties, of postsynaptic GABAA receptors. When taken together, our data indicate that nicotine exposure favors excitatory input directed to LDT cells from animals belonging to the younger age group; whereas, nicotine would be expected to activate a relatively larger proportion of inhibitory input directed to LDT cells from animals pertaining to the older age group. Furthermore, in the population of cells in which simultaneous excitatory and inhibitory miniature events were recorded, the drop out of nicotine mediated elicitation of mIPSCs with second applications while mEPSCs were robustly elicited in both exposures, indicates that nAChRs located on glutamatergic presynaptic terminals are less sensitive to nicotinic desensitization as compared to nAChRs located on GABAergic presynaptic terminals. Differential subunit compositions between nAChRs located on GABAergic and glutamatergic terminals could underlie the difference in sensitivity of mEPSCs and mIPSCs upon repeat exposures (Mansvelder et al., 2002; Tolu et al., 2013). Differences in subunit combinations of nAChRs located on GABAergic and glutamatergic terminals could therefore result in a greater degree of desensitization of nAChRs located on GABAergic terminals, which would induce a greater decrease of inhibitory as opposed to excitatory drive with a resultant excitatory effect.
The finding that the frequency of endogenous EPSCs was larger in LDT neurons in animals from the older as compared to the younger age group indicates that LDT neurons from younger animals experience reduced ongoing excitatory glutamatergic synaptic input suggesting the possibility of fewer active glutamatergic synapses in this age group. This was surprising since synapse pruning has been shown to initiate around postnatal week two in the mouse hippocampus (Liu et al., 2005; Stoneham et al., 2010) thereby possibly decreasing endogenous synaptic input at this later stage. However, activation of silent synapses which can occur within the first two to three postnatal weeks (Kerchner and Nicoll, 2008) and/or increases in synapse density that could lead to a heightened endogenous excitatory synaptic input within the first postnatal month have also been seen, which if occurring in the LDT could underlie the higher frequency of endogenous EPSCs found in cells from animals belonging to the older age group (Fiala et al., 1998; Harris et al., 1992). Interestingly, the larger amplitude of endogenous mIPSCs in LDT neurons in animals from the older age group would indicate the presence of a simultaneously larger endogenous inhibitory drive directed to LDT cells. The amplitude of mIPSCs depends upon the postsynaptic density and composition of GABAA receptors (Nusser et al., 1997; Simon et al., 2011), which have been shown to change in many brain areas across ontogeny (Lujan et al., 2005). Therefore, developmental increases in the postsynaptic density of GABAA receptors or developmental changes of GABAA subunits towards high affinity subunit composition in older animals could underlie the larger amplitude of endogenous mIPSCs in cells from group II animals. Additionally, across development a change in the chloride equilibrium potential, which changes GABA responses from excitatory to inhibitory within the first few postnatal days as the number of chloride transporters increases, occurs. The number of chloride transporters does, however, not reach adult levels until after approximately two weeks and until adult levels are reached, the amplitude of GABAergically-mediated mIPSCs would be expected to be smaller (Ben-Ari, 2002). Therefore, within the age range examined chloride transporters would be expected to further contribute to ontogenetic alterations in the amplitude of inhibitory chloride currents in intact cells.
4.04 Nicotine increases cellular excitability to a greater degree in P7-P15 when compared to P16-P34 animals
Our finding that nicotine induces a larger increase in the firing frequency of cholinergic LDT neurons from P7-P15 as compared to P16-P34 animals suggests that nicotine induces a functionally relevant larger excitatory effect on cells from animals belonging to the younger age group. By increasing neuronal firing frequency, nicotine would be expected to enhance neuronal output to target regions. Therefore, the larger nicotine-induced firing frequency in cells from younger animals could result in a differential cholinergic output from the LDT. Previous studies have shown that nicotine-mediated increases in the firing frequency of cholinergic LDT neurons are independent of the nicotinic induction of EPSCs and/or IPSCs (Ishibashi et al., 2009), indicating that increases depend upon postsynaptic, nicotine-mediated mechanisms. The persistence of a greater number of elicited spikes in LDT neurons from animals belonging to the younger age group in the presence of blockers of excitatory and inhibitory neurotransmitters further suggest that the difference in action potential firing frequency seen across development is also due to postsynaptic changes. The decrease found in the Ri across ontogeny could be a contributing factor to the heightened firing rate of cholinergic LDT neurons from younger animals. The larger Ri in LDT neurons from group I animals, which indicates reduced somatic conductances, serve to contribute relatively larger shifts in membrane potential at a given current input leading to reductions in current required to elicit action potentials. However, at comparable membrane depolarizations, larger firing frequencies were found in cells from P7-P15 when compared to P16-P34 animals, which suggests that postsynaptic mechanisms other than changes in Ri contribute to the ontogenetic changes in firing frequency.
In the present study, the broader nicotine-induced spike width seen in LDT neurons from P16-P34 animals could lead to the increase in the time period between spikes thereby contributing to the slower nicotine-induced firing frequency in animals from the older as compared to the younger age group. Although the remaining parameters examined of the action potential spike shape and AHP were not differentially affected by nicotine across ontogeny, the changes noted in spike shape and AHP across development could contribute to age-related differences in the effect of nicotine on the firing frequency. As nicotine induced a similar relative depolarization of the AHP minimum across both age groups, the more hyperpolarized AHP found in cells from animals belonging to the older age group would be expected to be relatively more affected by the nicotinic diminishment of the AHP minimum. In the older age group, the nicotine-induced depolarization of the AHP minimum could therefore contribute more to the nicotine-induced increase in cellular firing rate detected since a smaller AHP would decrease the action potential recovery period and thereby increase the firing frequency. However, complicating this interpretation are findings that decreases in AHP minimums have been shown to reduce cell output due to a reduction of removal of sodium channel inactivation (Erisir et al., 1999). Therefore, ontogenetic differences in kinetics of voltage dependent sodium channels may also play a role in the functional outcome of nicotine effects on AHP minimums. The AHP minimum is greatly dependent upon the initial fast AHP, which has been found to be mediated by a large conductance calcium-activated potassium (BK) channel in the LDT (Kohlmeier et al., 2013) and shown in other cells types to be affected by nicotine (Kuhlmann et al., 2005). If similar actions on BK channels are present in the LDT, nicotinic inhibition of BK channels could subsequently underlie the nicotine-induced depolarization of the AHP minimum. Increases in the number of BK channels across age shown in the pons (Macdonald et al., 2006) could contribute to the more hyperpolarized AHP found in cholinergic LDT cells in animals from the older age group. The larger and more hyperpolarized AHP found in cholinergic LDT cells from the older age group would be expected to contribute to the developmental decrease seen in the evoked firing frequency of cholinergic cells by increasing the action potential recovery rate. While the developmental changes found in the AHP would be expected to decrease cellular excitability in cholinergic LDT neurons from animals pertaining to the older age group, the ontogenetic associated reduction in the membrane potential threshold required for action potential firing would be expected to lead to a greater cellular excitability in these cells as a smaller depolarization would be required to reach firing threshold. Similarly the narrowing of the spike width across development which was also observed in neurons of the PPT (Kobayashi et al., 2004) would decrease the time duration between spikes in cholinergic cells from P16-P34 animals thereby contributing to a greater excitability. The broader spike width seen in P7-P15 animals would, however, be expected to increase action potential-mediated rises in intracellular calcium thereby possibly producing a larger effect on intracellular calcium dependent mechanisms.
4.05 Functional Significance:
The ontogenetic changes found in nicotine's effect on cholinergic LDT neurons could indicate that upon nicotine exposure, cells from younger animals would be excited to a greater extent, and more likely to fire, which would suggest a larger output to target areas. Cholinergic neurons of the LDT send diffuse projections both caudally and rostrally, and are via these projections involved in diverse behaviors. Functionally-relevant connectivity of the LDT to the VTA has been established (Forster and Blaha, 2000; Lammel et al., 2012; Lodge and Grace, 2006) and ACh-containing LDT neurons provide the major cholinergic input to DA-containing, mesoaccumbal projecting VTA neurons (Oakman et al., 1995; Omelchenko and Sesack, 2005; Omelchenko and Sesack, 2006). Further, the asymmetric nature of these cholinergic synapses (Omelchenko and Sesack, 2006) and the role of endogenous ACh in increasing firing of DA-VTA cells (Mameli-Engvall et al., 2006; Melis et al., 2013) indicate that LDT-mediated cholinergic input to DA-VTA cells is excitatory. Activation of the mesoaccumbal pathway including excitation of DA-VTA cells plays a deterministic role in development of drug dependency (Corrigall et al., 1994; Corrigall et al., 1992) and input from the LDT influences the excitability of DA-VTA neurons (Lammel et al., 2012). Supporting a critical role of the LDT in mesoaccumbal activity is the finding that ablation of the LDT eliminates the firing pattern believed to be behaviorally relevant in addiction processes (Lodge and Grace, 2006). The ACh released from LDT terminals within the VTA is believed to act at nAChRs located on DA-VTA neurons as well as GABA- and glutamate-containing VTA terminals (Mameli-Engvall et al., 2006; Schilstrom et al., 2003). Ontogenetic differences in nicotine-induced excitability in the LDT, suggesting an increase in ACh release in the VTA might be less biologically relevant in promoting age-related differences of activation via this mechanism, since nicotinic activation of nAChRs located within the VTA are believed to rapidly lead to desensitization (Mansvelder et al., 2002; Pidoplichko et al., 1997), rendering them unavailable to activate following endogenous ACh release (Mansvelder et al., 2002). However, excitatory muscarinic receptors within the VTA (Gronier and Rasmussen, 1998; Lacey et al., 1990) are believed to play an instrumental role in LDT-related addiction processes (Blaha et al., 1996; Forster and Blaha, 2000; Miller and Blaha, 2005) as muscarinic blockade in the VTA results in attenuation of addiction-related behaviors (Ikemoto and Wise, 2002). While this action could be mediated in part via blockade of autoinhibitory M4 receptors located on LDT terminals in the VTA (Tzavara et al., 2004), muscarinic activation in the VTA is strongly rewarding and animals will self-administer muscarinic receptor agonists, suggesting that the net result of muscarinic activation in the VTA is excitatory (Ikemoto and Wise, 2002; Yeomans et al., 1985). Rewarding actions of muscarinic agents within the VTA are believed to be due in large part to a long-lived excitation of VTA-located M5 receptors, which is the only muscarinic subtype definitively identified on DA-VTA neurons (Vilaro et al., 1990). Atropine-induced actions are missing in animals in which the M5 receptor has been genetically deleted or inactivated via VTA infusions of M5 antisense and behavioral studies have shown that animals lacking functioning M5 receptors exhibit a reduction in DA transmission and alterations in thresholds for reward (Forster et al., 2002; Yeomans et al., 2000). Therefore, the ontogenetic differences in the nAChR excitation of cholinergic LDT neurons could result in differential nicotine-mediated release of ACh to target structures within the VTA, which while it may have a negligible action at quickly desensitizing nAChRs on VTA cells, could induce a heightened activation of M5 receptors located on DA-VTA neurons, resulting in enhanced DA release in NAcc targets in younger animals. As levels of DA release in this circuit correlate with experience of euphoria (Drevets et al., 2001; Pontieri et al., 1995; Pontieri et al., 1996), such a heighted release would be expected to confer a greater degree of reward to use of nicotine in younger individuals. Accordingly, a greater degree of nicotine-induced excitability in cholinergic LDT neurons demonstrated in the present study could play a part in the higher susceptibility to nicotine addiction seen in younger individuals. It will be of interest to determine whether the decrease in nicotinic-mediated cholinergic cellular excitability noted in the ranges of ages examined demonstrates the same trajectory across a larger range of ontogenetic periods, which may explain why initiation of smoking at an early age is associated with a high risk of development of nicotine dependence, whereas exposure to nicotine at older ages is less likely to lead to addiction (Chen and Millar, 1998; Kendler et al., 2013; Wilkinson et al., 2007).
5. Conclusion
The difference in the susceptibility to addict to nicotine between adolescents and adults is well established, but mechanisms in the reward pathway, which may underlie the developmental differences, have not been thoroughly elucidated. In this study we found neuronal changes in response to nicotine across ontogeny in cholinergic neurons importantly involved in the mediation of reward which could contribute to the differential susceptibility to addict to nicotine across ontogeny. Future studies examining whether differential changes also occur in LDT cells across ontogeny in response to long term nicotine exposure are important to further increase our knowledge about the differential ontogenetic effects of nicotine underlying the differential susceptibility to addict to this drug seen across age. In combination with the information obtained from the present study, such knowledge will assist in the development of age specific therapeutical treatments to aid smoking cessation.
Highlights.
Nicotine induces larger rises in calcium in LDT neurons from younger animals.
Nicotine induces larger membrane currents in LDT neurons from younger animals
Nicotine induces greater excitatory synaptic activity in LDT neurons from younger animals.
Acknowledgments
The authors would like to thank Mr. Jason Allen Teem and Dr. Iryna Gumenchuk for their assistance with the immunohistochemical work presented in this manuscript. Laura K.F. McNair is thanked for her contribution of data added upon revision.
Funding sources: This work was funded partly by a research grant from the Philip Morris External Research Program (K.A.K), National Institutes of Health grants NS27881 (C.S.L), HL64150 (C.S.L), and funds from the University of Copenhagen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors disclose that they have no conflict of interest with respect to this manuscript
Reference List
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J Pharmacol Exp Ther. 1993;265:1455–1473. [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Wonnacott S, Albuquerque EX. Blockade of nicotinic currents in hippocampal neurons defines methyllycaconitine as a potent and specific receptor antagonist. Mol Pharmacol. 1992;41:802–808. [PubMed] [Google Scholar]
- American Psychiatric Association D-V Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. 2013. DSM-V.
- Azam L, Chen Y, Leslie FM. Developmental regulation of nicotinic acetylcholine receptors within midbrain dopamine neurons. Neuroscience. 2007;144:1347–1360. doi: 10.1016/j.neuroscience.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan U, Leslie FM. Co-expression of alpha7 and beta2 nicotinic acetylcholine receptor subunit mRNAs within rat brain cholinergic neurons. Neuroscience. 2003;119:965–977. doi: 10.1016/s0306-4522(03)00220-3. [DOI] [PubMed] [Google Scholar]
- Baumgold J, Zimmerman I, Bambrick L. Appearance of [3H]saxitoxin binding sites in developing rat brain. Brain Res. 1983;285:405–407. doi: 10.1016/0165-3806(83)90040-8. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Biel M, Wahl-Schott C, Michalakis S, Zong X. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev. 2009;89:847–885. doi: 10.1152/physrev.00029.2008. [DOI] [PubMed] [Google Scholar]
- Blaha CD, Allen LF, Das S, Inglis WL, Latimer MP, Vincent SR, Winn P. Modulation of dopamine efflux in the nucleus accumbens after cholinergic stimulation of the ventral tegmental area in intact, pedunculopontine tegmental nucleus-lesioned, and laterodorsal tegmental nucleus-lesioned rats. J Neurosci. 1996;16:714–722. doi: 10.1523/JNEUROSCI.16-02-00714.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucetta S, Jones BE. Activity profiles of cholinergic and intermingled GABAergic and putative glutamatergic neurons in the pontomesencephalic tegmentum of urethane-anesthetized rats. J Neurosci. 2009;29:4664–4674. doi: 10.1523/JNEUROSCI.5502-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Millar WJ. Age of smoking initiation: implications for quitting. Health Rep. 1998;9:39–46. [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Couraud F, Martin-Moutot N, Koulakoff A, Berwald-Netter Y. Neurotoxin-sensitive sodium channels in neurons developing in vivo and in vitro. J Neurosci. 1986;6:192–198. doi: 10.1523/JNEUROSCI.06-01-00192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautan D, Huerta-Ocampo I, Witten IB, Deisseroth K, Bolam JP, Gerdjikov T, Mena-Segovia J. A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the brainstem. J Neurosci. 2014;34:4509–4518. doi: 10.1523/JNEUROSCI.5071-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR. Hooked from the first cigarette. Sci Am. 2008;298:82–87. doi: 10.1038/scientificamerican0508-82. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Rigotti NA, McNeill AD, Ockene JK, Savageau JA, St CD, Coleman M. Initial symptoms of nicotine dependence in adolescents. Tob Control. 2000;9:313–319. doi: 10.1136/tc.9.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Sweet M, Savageau J, Ursprung WW. An evaluation of a clinical approach to staging tobacco addiction. J Pediatr. 2011;159:999–1003. doi: 10.1016/j.jpeds.2011.05.037. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2010;72:114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Price JL, Mathis CA. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacol Ther. 2009;122:125–139. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisir A, Lau D, Rudy B, Leonard CS. Function of specific K(+) channels in sustained high-frequency firing of fast-spiking neocortical interneurons. J Neurophysiol. 1999;82:2476–2489. doi: 10.1152/jn.1999.82.5.2476. [DOI] [PubMed] [Google Scholar]
- Fenster CP, Rains MF, Noerager B, Quick MW, Lester RA. Influence of subunit composition on desensitization of neuronal acetylcholine receptors at low concentrations of nicotine. J Neurosci. 1997;17:5747–5759. doi: 10.1523/JNEUROSCI.17-15-05747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci. 1998;18:8900–8911. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Forster GL, Blaha CD. Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur J Neurosci. 2000;12:3596–3604. doi: 10.1046/j.1460-9568.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- Forster GL, Yeomans JS, Takeuchi J, Blaha CD. M5 muscarinic receptors are required for prolonged accumbal dopamine release after electrical stimulation of the pons in mice. J Neurosci. 2002;22:RC190. doi: 10.1523/JNEUROSCI.22-01-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Jin Y, Yang K, Zhang D, Lukas RJ, Wu J. Mechanisms involved in systemic nicotine-induced glutamatergic synaptic plasticity on dopamine neurons in the ventral tegmental area. J Neurosci. 2010;30:13814–13825. doi: 10.1523/JNEUROSCI.1943-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CH, Bay KD, Buchanan RA, McKeon KA, Skinner RD, Garcia-Rill E. Prenatal exposure to cigarette smoke affects the physiology of pedunculopontine nucleus (PPN) neurons in development. Neurotoxicol Teratol. 2006;28:210–219. doi: 10.1016/j.ntt.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Grace AA, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989;9:3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griguoli M, Maul A, Nguyen C, Giorgetti A, Carloni P, Cherubini E. Nicotine blocks the hyperpolarization-activated current Ih and severely impairs the oscillatory behavior of oriens-lacunosum moleculare interneurons. J Neurosci. 2010;30:10773–10783. doi: 10.1523/JNEUROSCI.2446-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronier B, Rasmussen K. Activation of midbrain presumed dopaminergic neurones by muscarinic cholinergic receptors: an in vivo electrophysiological study in the rat. Br J Pharmacol. 1998;124:455–464. doi: 10.1038/sj.bjp.0701850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard JR, Hamill OP, Prince DA. Developmental changes in Na+ conductances in rat neocortical neurons: appearance of a slowly inactivating component. J Neurophysiol. 1988;59:778–795. doi: 10.1152/jn.1988.59.3.778. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Wise RA. Rewarding effects of the cholinergic agents carbachol and neostigmine in the posterior ventral tegmental area. J Neurosci. 2002;22:9895–9904. doi: 10.1523/JNEUROSCI.22-22-09895.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi M, Leonard CS, Kohlmeier KA. Nicotinic activation of laterodorsal tegmental neurons: implications for addiction to nicotine. Neuropsychopharmacology. 2009;34:2529–2547. doi: 10.1038/npp.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia HG, Yamuy J, Sampogna S, Morales FR, Chase MH. Colocalization of gamma-aminobutyric acid and acetylcholine in neurons in the laterodorsal and pedunculopontine tegmental nuclei in the cat: a light and electron microscopic study. Brain Res. 2003;992:205–219. doi: 10.1016/j.brainres.2003.08.062. [DOI] [PubMed] [Google Scholar]
- Kanyshkova T, Pawlowski M, Meuth P, Dube C, Bender RA, Brewster AL, Baumann A, Baram TZ, Pape HC, Budde T. Postnatal expression pattern of HCN channel isoforms in thalamic neurons: relationship to maturation of thalamocortical oscillations. J Neurosci. 2009;29:8847–8857. doi: 10.1523/JNEUROSCI.0689-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai H, Lazar R, Metherate R. Nicotinic control of axon excitability regulates thalamocortical transmission. Nat Neurosci. 2007;10:1168–1175. doi: 10.1038/nn1956. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Damaj MI, Chen X. Early smoking onset and risk for subsequent nicotine dependence: a monozygotic co-twin control study. Am J Psychiatry. 2013;170:408–413. doi: 10.1176/appi.ajp.2012.12030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9:813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Good C, Biedermann J, Barnes C, Skinner RD, Garcia-Rill E. Developmental changes in pedunculopontine nucleus (PPN) neurons. J Neurophysiol. 2004;91:1470–1481. doi: 10.1152/jn.01024.2003. [DOI] [PubMed] [Google Scholar]
- Kohlmeier KA, Christensen MH, Kristensen MP, Kristiansen U. Pharmacological evidence of functional inhibitory metabotrophic glutamate receptors on mouse arousal-related cholinergic laterodorsal tegmental neurons. Neuropharmacology. 2013;66:99–113. doi: 10.1016/j.neuropharm.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Kohlmeier KA, Inoue T, Leonard CS. Hypocretin/orexin peptide signaling in the ascending arousal system: elevation of intracellular calcium in the mouse dorsal raphe and laterodorsal tegmentum. J Neurophysiol. 2004;92:221–235. doi: 10.1152/jn.00076.2004. [DOI] [PubMed] [Google Scholar]
- Kristensen MP, Leonard CS. (Electrophysiological properties of laterodorsal tegmental nucleus (LDT) neurons from the developing mouse: Excitability, typology, nitric oxide synthase (bNOS) immunoreactivity and the relation to published data from other species).. Society for Neuroscience Annual Meeting; San Diego, USA. November 2007. [Google Scholar]
- Kuhlmann CR, Trumper JR, Tillmanns H, Alexander SC, Erdogan A. Nicotine inhibits large conductance Ca(2+)-activated K(+) channels and the NO/-cGMP signaling pathway in cultured human endothelial cells. Scand Cardiovasc J. 2005;39:348–352. doi: 10.1080/14017430500200465. [DOI] [PubMed] [Google Scholar]
- Kurosawa R, Taoka N, Shinohara F, Minami M, Kaneda K. Cocaine exposure enhances excitatory synaptic drive to cholinergic neurons in the laterodorsal tegmental nucleus. Eur J Neurosci. doi: 10.1111/ejn.12296. doi: 10.1111epub ahead of print, 2013. [DOI] [PubMed] [Google Scholar]
- Lacey MG, Calabresi P, North RA. Muscarine depolarizes rat substantia nigra zona compacta and ventral tegmental neurons in vitro through M1-like receptors. J Pharmacol Exp Ther. 1990;253:395–400. [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhu W, Zhang ZS, Yang T, Grant A, Oxford G, Simon SA. Nicotine inhibits voltage-dependent sodium channels and sensitizes vanilloid receptors. J Neurophysiol. 2004;91:1482–1491. doi: 10.1152/jn.00922.2003. [DOI] [PubMed] [Google Scholar]
- Liu XB, Low LK, Jones EG, Cheng HJ. Stereotyped axon pruning via plexin signaling is associated with synaptic complex elimination in the hippocampus. J Neurosci. 2005;25:9124–9134. doi: 10.1523/JNEUROSCI.2648-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci U S A. 2006;103:5167–5172. doi: 10.1073/pnas.0510715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan R, Shigemoto R, Lopez-Bendito G. Glutamate and GABA receptor signalling in the developing brain. Neuroscience. 2005;130:567–580. doi: 10.1016/j.neuroscience.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Ma L, Wu YM, Guo YY, Yang Q, Feng B, Song Q, Liu SB, Zhao DQ, Zhao MG. Nicotine addiction reduces the large-conductance Ca(2+)-activated potassium channels expression in the nucleus accumbens. Neuromolecular Med. 2013;15:227–237. doi: 10.1007/s12017-012-8213-y. [DOI] [PubMed] [Google Scholar]
- Macdonald SH, Ruth P, Knaus HG, Shipston MJ. Increased large conductance calcium-activated potassium (BK) channel expression accompanied by STREX variant downregulation in the developing mouse CNS. BMC Dev Biol. 2006;6:37. doi: 10.1186/1471-213X-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay J, Crofton J. Tobacco and the developing world. Br Med Bull. 1996;52:206–221. doi: 10.1093/oxfordjournals.bmb.a011527. [DOI] [PubMed] [Google Scholar]
- Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux JP, Faure P. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron. 2006;50:911–921. doi: 10.1016/j.neuron.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Post-natal development of electrophysiological properties of rat cerebral cortical pyramidal neurones. J Physiol. 1987;393:743–762. doi: 10.1113/jphysiol.1987.sp016851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Scheggi S, Carta G, Madeddu C, Lecca S, Luchicchi A, Cadeddu F, Frau R, Fattore L, Fadda P, Ennas MG, Castelli MP, Fratta W, Schilstrom B, Banni S, De Montis MG, Pistis M. PPARalpha regulates cholinergic-driven activity of midbrain dopamine neurons via a novel mechanism involving alpha7 nicotinic acetylcholine receptors. J Neurosci. 2013;33:6203–6211. doi: 10.1523/JNEUROSCI.4647-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Liu C, Bishop K, Gong ZH, Nordberg A, Zhang X. Nicotine exposure during a critical period of development leads to persistent changes in nicotinic acetylcholine receptors of adult rat brain. J Neurochem. 1998;70:752–762. doi: 10.1046/j.1471-4159.1998.70020752.x. [DOI] [PubMed] [Google Scholar]
- Mieda M, Hasegawa E, Kisanuki YY, Sinton CM, Yanagisawa M, Sakurai T. Differential roles of orexin receptor-1 and -2 in the regulation of non-REM and REM sleep. J Neurosci. 2011;31:6518–6526. doi: 10.1523/JNEUROSCI.6506-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AD, Blaha CD. Midbrain muscarinic receptor mechanisms underlying regulation of mesoaccumbens and nigrostriatal dopaminergic transmission in the rat. Eur J Neurosci. 2005;21:1837–1846. doi: 10.1111/j.1460-9568.2005.04017.x. [DOI] [PubMed] [Google Scholar]
- Nelson CL, Wetter JB, Milovanovic M, Wolf ME. The laterodorsal tegmentum contributes to behavioral sensitization to amphetamine. Neuroscience. 2007;146:41–49. doi: 10.1016/j.neuroscience.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. Alternate stoichiometries of alpha4beta2 nicotinic acetylcholine receptors. Mol Pharmacol. 2003;63:332–341. doi: 10.1124/mol.63.2.332. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Kayama Y, Koyama Y. Postnatal development of cholinergic neurons in the mesopontine tegmentum revealed by histochemistry. Int J Dev Neurosci. 2005;23:711–721. doi: 10.1016/j.ijdevneu.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Cull-Candy S, Farrant M. Differences in synaptic GABA(A) receptor number underlie variation in GABA mini amplitude. Neuron. 1997;19:697–709. doi: 10.1016/s0896-6273(00)80382-7. [DOI] [PubMed] [Google Scholar]
- Nutter TJ, Adams DJ. Monovalent and divalent cation permeability and block of neuronal nicotinic receptor channels in rat parasympathetic ganglia. J Gen Physiol. 1995;105:701–723. doi: 10.1085/jgp.105.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK. Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J Neurosci. 1995;15:5859–5869. doi: 10.1523/JNEUROSCI.15-09-05859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Laterodorsal tegmental projections to identified cell populations in the rat ventral tegmental area. J Comp Neurol. 2005;483:217–235. doi: 10.1002/cne.20417. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Cholinergic axons in the rat ventral tegmental area synapse preferentially onto mesoaccumbens dopamine neurons. J Comp Neurol. 2006;494:863–875. doi: 10.1002/cne.20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, Noguchi J, Areola OO, Liang Y, Peterson J, Zhang T, Dani JA. Nicotinic cholinergic synaptic mechanisms in the ventral tegmental area contribute to nicotine addiction. Learn Mem. 2004;11:60–69. doi: 10.1101/lm.70004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placzek AN, Zhang TA, Dani JA. Age dependent nicotinic influences over dopamine neuron synaptic plasticity. Biochem Pharmacol. 2009;78:686–692. doi: 10.1016/j.bcp.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di CG. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di CG. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Quick MW, Lester RA. Desensitization of neuronal nicotinic receptors. J Neurobiol. 2002;53:457–478. doi: 10.1002/neu.10109. [DOI] [PubMed] [Google Scholar]
- Ramoa AS, McCormick DA. Developmental changes in electrophysiological properties of LGNd neurons during reorganization of retinogeniculate connections. J Neurosci. 1994;14:2089–2097. doi: 10.1523/JNEUROSCI.14-04-02089.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Rawal N, Mameli-Engvall M, Nomikos GG, Svensson TH. Dual effects of nicotine on dopamine neurons mediated by different nicotinic receptor subtypes. Int J Neuropsychopharmacol. 2003;6:1–11. doi: 10.1017/S1461145702003188. [DOI] [PubMed] [Google Scholar]
- Scragg R, Wellman RJ, Laugesen M, DiFranza JR. Diminished autonomy over tobacco can appear with the first cigarettes. Addict Behav. 2008;33:689–698. doi: 10.1016/j.addbeh.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Shen JX, Yakel JL. Nicotinic acetylcholine receptor-mediated calcium signaling in the nervous system. Acta Pharmacol Sin. 2009;30:673–680. doi: 10.1038/aps.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JD, Elash C. Nicotine withdrawal in chippers and regular smokers: subjective and cognitive effects. Health Psychol. 1995;14:301–309. doi: 10.1037//0278-6133.14.4.301. [DOI] [PubMed] [Google Scholar]
- Simon C, Hayar A, Garcia-Rill E. Responses of developing pedunculopontine neurons to glutamate receptor agonists. J Neurophysiol. 2011;105:1918–1931. doi: 10.1152/jn.00953.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoneham ET, Sanders EM, Sanyal M, Dumas TC. Rules of engagement: factors that regulate activity-dependent synaptic plasticity during neural network development. Biol Bull. 2010;219:81–99. doi: 10.1086/BBLv219n2p81. [DOI] [PubMed] [Google Scholar]
- Tolu S, Eddine R, Marti F, David V, Graupner M, Pons S, Baudonnat M, Husson M, Besson M, Reperant C, Zemdegs J, Pages C, Hay YA, Lambolez B, Caboche J, Gutkin B, Gardier AM, Changeux JP, Faure P, Maskos U. Co-activation of VTA DA and GABA neurons mediates nicotine reinforcement. Mol Psychiatry. 2013;18:382–393. doi: 10.1038/mp.2012.83. [DOI] [PubMed] [Google Scholar]
- Tsien RY. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981;290:527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Bymaster FP, Davis RJ, Wade MR, Perry KW, Wess J, McKinzie DL, Felder C, Nomikos GG. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 2004;18:1410–1412. doi: 10.1096/fj.04-1575fje. [DOI] [PubMed] [Google Scholar]
- Vasilyev DV, Barish ME. Postnatal development of the hyperpolarization-activated excitatory current Ih in mouse hippocampal pyramidal neurons. J Neurosci. 2002;22:8992–9004. doi: 10.1523/JNEUROSCI.22-20-08992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilaro MT, Palacios JM, Mengod G. Localization of m5 muscarinic receptor mRNA in rat brain examined by in situ hybridization histochemistry. Neurosci Lett. 1990;114:154–159. doi: 10.1016/0304-3940(90)90064-g. [DOI] [PubMed] [Google Scholar]
- Vincent SR, Kimura H. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience. 1992;46:755–784. doi: 10.1016/0306-4522(92)90184-4. [DOI] [PubMed] [Google Scholar]
- Vincent SR, Satoh K, Armstrong DM, Fibiger HC. NADPH-diaphorase: a selective histochemical marker for the cholinergic neurons of the pontine reticular formation. Neurosci Lett. 1983;43:31–36. doi: 10.1016/0304-3940(83)90124-6. [DOI] [PubMed] [Google Scholar]
- Wang H, Shi H, Wang Z. Nicotine depresses the functions of multiple cardiac potassium channels. Life Sci. 1999;65:L143–L149. doi: 10.1016/s0024-3205(99)00370-7. [DOI] [PubMed] [Google Scholar]
- Wang H, Shi H, Zhang L, Pourrier M, Yang B, Nattel S, Wang Z. Nicotine is a potent blocker of the cardiac A-type K(+) channels. Effects on cloned Kv4.3 channels and native transient outward current. Circulation. 2000a;102:1165–1171. doi: 10.1161/01.cir.102.10.1165. [DOI] [PubMed] [Google Scholar]
- Wang H, Yang B, Zhang L, Xu D, Wang Z. Direct block of inward rectifier potassium channels by nicotine. Toxicol Appl Pharmacol. 2000b;164:97–101. doi: 10.1006/taap.2000.8896. [DOI] [PubMed] [Google Scholar]
- Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson AV, Schabath MB, Prokhorov AV, Spitz MR. Age-related differences in factors associated with smoking initiation. Cancer Causes Control. 2007;18:635–644. doi: 10.1007/s10552-007-9008-6. [DOI] [PubMed] [Google Scholar]
- Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci. 2003;23:3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans JS, Kofman O, McFarlane V. Cholinergic involvement in lateral hypothalamic rewarding brain stimulation. Brain Res. 1985;329:19–26. doi: 10.1016/0006-8993(85)90508-6. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Takeuchi J, Baptista M, Flynn DD, Lepik K, Nobrega J, Fulton J, Ralph MR. Brain-stimulation reward thresholds raised by an antisense oligonucleotide for the M5 muscarinic receptor infused near dopamine cells. J Neurosci. 2000;20:8861–8867. doi: 10.1523/JNEUROSCI.20-23-08861.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, Verbny Y, Malek SA, Stys PK, Chiu SY. Nicotinic acetylcholine receptors in mouse and rat optic nerves. J Neurophysiol. 2004;91:1025–1035. doi: 10.1152/jn.00769.2003. [DOI] [PubMed] [Google Scholar]
- Zhang X, Liu C, Miao H, Gong ZH, Nordberg A. Postnatal changes of nicotinic acetylcholine receptor alpha 2, alpha 3, alpha 4, alpha 7 and beta 2 subunits genes expression in rat brain. Int J Dev Neurosci. 1998;16:507–518. doi: 10.1016/s0736-5748(98)00044-6. [DOI] [PubMed] [Google Scholar]