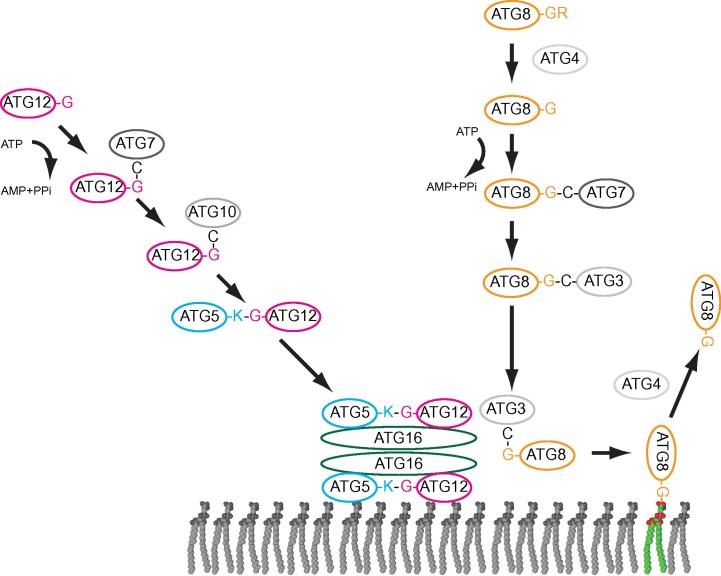
Fig. 1.
The Atg8 and Atg12 conjugation machinery. S. cerevisiae Atg8 is initially synthesized with a C-terminal arginine residue (R117). The cysteine protease Atg4 mediates the cleavage of this C-terminal arginine exposing the penultimate glycine, which becomes activated by the E1-like enzyme Atg7 under consumption of ATP. From Atg7 Atg8 becomes transferred to a cysteine in the E2-like Atg3. From there Atg8 is transferred to the headgroup of the membrane lipid phosphatidylethanolamine. Alternatively, phosphatidylserine can serve as an acceptor of Atg8. The transfer of Atg8 to the lipid headgroup is massively stimulated by a complex composed of the Atg5, Atg12 and Atg16 proteins. This complex is itself the result of an ubiquitin-like conjugation reaction. Here the C-terminus of the ubiquitin-like Atg12 is covalently attached to a lysine residue (K149) of the Atg5 protein. Atg7 and the E2-like Atg10 protein mediate this conjugation. The resulting Atg12–Atg5 conjugate forms a complex with the Atg16 protein giving rise to the Atg12–Atg5-Atg16 complex. Atg4, the same enzyme that mediates the removal of R117 from Atg8, is able to remove Atg8 from the membrane by hydrolyzing the bond between the C-terminal glycine and the lipid headgroup.