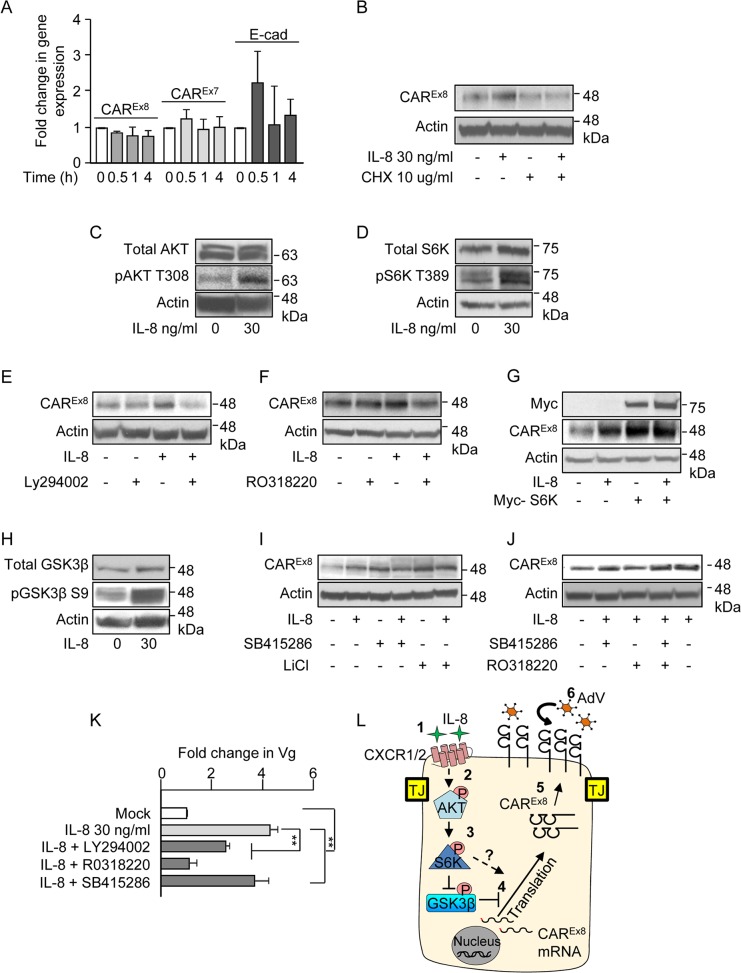
Fig 6. IL-8 activates AKT/S6K and inactivates GSK3β to increase CAREx8 protein synthesis and AdV entry.
A) The apical surfaces of polarized primary airway epithelial cells were either mock (0, white bars) or IL-8 (30 ng/ml, gray bars) treated for the indicated time and analyzed for CAREx8, CAREx7, or E-cadherin (E-cad) gene expression by qPCR, relative to GAPDH. B) The apical surfaces of polarized primary airway epithelial cells were mock (0) or IL-8 treated in the presence or absence of cycloheximide (CHX) and lysates were analyzed for CAREx8 and actin protein expression. Activation state of C) AKT, D) S6K and H) GSK3β was analyzed after IL-8 treatment by probing for the pAKT T308, pS6K T389, and pGSK3β S9 respectively. Lysates from polarized cells treated with IL-8 in the presence or absence of chemical inhibitors for E) AKT (Ly294002, 30 μM), F) S6K (RO3118220, 300 nM), I) GSK3β (SB415286, 45 μM, or LiCl, 10 mM), or J) a combination of S6K (RO3118220, 300 nM) and GSK3β (SB415286, 45 μM) were investigated for CAREx8 and actin protein expression. G) Polarized cells were either transfected or not with myc-tagged S6K plasmid prior to mock (0) or IL-8 treatment followed by the analysis of CAREx8 and actin protein expression from cell lysates. K) Polarized cells exposed to IL-8 in the presence or absence of the indicated chemical inhibitors for 4 h were washed and transduced with AdV5-βGal for 1 h. Genomic DNA was isolated 24 h post-transduction and analyzed for the fold change in Vg normalized to GAPDH and relative to mock. Error bars represent the SEM from three independent experiments: **p < 0.001 by one way ANOVA and Bonferroni post hoc test. L) A schematic of a predicted model showing that 1) IL-8 binds to the IL-8 receptor (CXCR1/2) and 2) activates AKT. 3) Activated AKT (pAKT T308) further activates S6K (pS6K T389) and 4) activated AKT directly and/or via inhibition of GSK3β (pGSK3β S9) stimulates CAREx8 protein synthesis. 5) Newly synthesized CAREx8 traffics to the apical surface and 6) can mediate apical AdV infection.