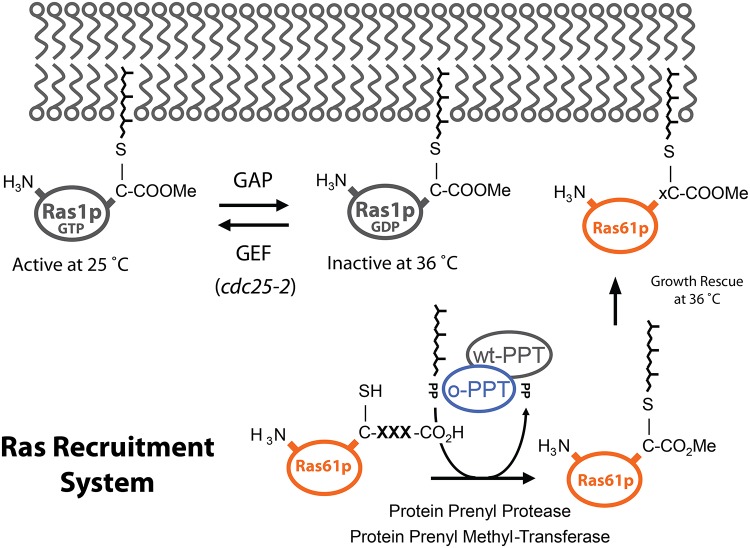
Fig 1. The principle of the Ras Recruitment System (RRS).
The system is based on a temperature sensitive GDP exchange factor (encoded by the cdc25–2 allele) that is rendered inactive at 36°C trapping endogenous Ras1p in its inactive GDP bound form. Growth is rescued by genetic complementation with a constitutively active mutant of mammalian H-Ras (RAS61). To exert its function and rescue growth, Ras61p needs to be directed to the plasma membrane. This can either occur through protein-protein interactions or lipid modifications such as myristoylation or prenylation. Specifically, prenylation can either be mediated by endogenous protein prenyltransferases (wt-PPTases) that recognise naturally occurring, prenylatable CaaX-box motives or engineered protein prenyltransferases (o-PPTases) that recognise orthogonal CaaX-box motives that are not recognised by the endogenous machinery. For optimal membrane recruitment and genetic complementation in the RRS, the three most C-terminal amino acids of prenylated CaaX-box motives are removed by highly specific protein prenyl proteases located in the endoplasmic reticulum followed by carboxymethylesterification of the C-terminus.