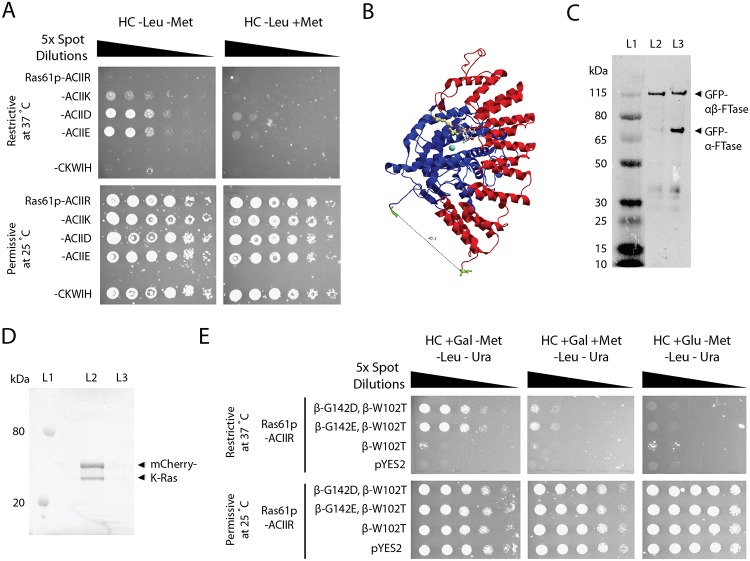
Fig 4. Engineering FTases with altered substrate specificities.
(A) CaaX-box motives with positively charged residues in the anchoring position X cannot rescue growth in the RRS and thus provide poor substrates for endogenous FTases in Saccharomyces cerevisiae. (B) Structural model of the αβ-FTase heterodimer derived from Rattus norvegicus (PDB: 1KZO). The C-terminus of the α-subunit (highlighted in blue) is separated by 40 Å from the N-terminus of the β-subunit (highlighted in red). (C) Western blot analysis of GFP-αβ-FTase fusion proteins derived from R. norvegicus expressed in Leishmania tarantolae cell-free expression system. The linker connecting α- and β-subunits contained a TEV protease cleavage site that is cleaved with exogenously added TEV protease. L1: Protein Ladder; L2: Uncleaved GFP-αβ-FTase; L3: GFP-αβ-FTase cleaved with TEV Protease. (D) Fluorescent scan of SDS–PAGE loaded with mCherry-K-Ras in vitro prenylation reaction containing single-chain GFP-αβ-FTase fusion proteins and fluorescent phosphoisoprenoid NBD-GPP [35]. Addition of FPP to the reaction prevents formation of the fluorescent reaction product due to competition with the fluorescent lipid donor. L1: Protein Ladder; L2: GFP-αβ-FTase bound to GFP-Cap beads, 5 μM mCherry-K-Ras, 5 μM NBP-GPP; L3: GFP-αβ-FTase bound to GFP-Cap beads, 5 μM mCherry-K-Ras, 5 μM NBD-GPP, 25 μM FPP. (E) To facilitate expression and prevent cross-heterodimerisation between yeast and exogenous FTase subunits, a single-chain αβ-FTase was created based on mutant β-W102T while introducing negative charges at the bottom of the active site at β-G142D and β-G142E enabling FTase to farnesylate a CaaX-box motif with a positive charge in X and thus rescue growth in the RRS. Controls: pYES2 denotes vector control and β-W102T the unmodified, single-chain αβ-FTaseβ-W102T mutant neither of which can prenylate the orthogonal CaaX-box motif.