Abstract
Clinical data relating to rabbit antithymocyte globulin (rATG) induction in heart transplantation are far less extensive than for other immunosuppressants, or indeed for rATG in other indications. This was highlighted by the low grade of evidence and the lack of detailed recommendations for prescribing rATG in the International Society for Heart and Lung Transplantation (ISHLT) guidelines. The heart transplant population includes an increasing frequency of patients on mechanical circulatory support (MCS), often with ongoing infection and/or presensitization, who are at high immunological risk but also vulnerable to infectious complications. The number of patients with renal impairment is also growing due to lengthening waiting times, intensifying the need for strategies that minimize calcineurin inhibitor (CNI) toxicity. Additionally, the importance of donor-specific antibodies (DSA) in predicting graft failure is influencing immunosuppressive regimens. In light of these developments, and in view of the lack of evidence-based prescribing criteria, experts from Germany, Austria, and Switzerland convened to identify indications for rATG induction in heart transplantation and to develop an algorithm for its use based on patient characteristics.
Keywords: antithymocyte globulin, heart transplantation, rabbit antithymocyte globulin, Thymoglobulin
Introduction
Heart transplant recipients frequently receive induction with rabbit antithymocyte globulin (rATG; Thymoglobulin®, Genzyme Corporation, Cambridge, MA, USA). Overall, approximately a fifth of all adult heart transplant patients in the US [1,2] and 30% of patients in Europe [2] receive rATG induction, and approximately half of all pediatric recipients [3], but its use varies widely between countries and between centers. This is partly due to the fact that although rATG has been licensed for 30 years, there is still a remarkable paucity of well-conducted trials examining its efficacy and safety in solid organ transplantation. Most of the available studies have been carried out in kidney transplant populations. Heart transplantation, however, represents unique challenges that mean data from other organ types are not necessary applicable. Heart transplant recipients are generally in a more unstable condition and often maintained on mechanical circulatory support (MCS) devices; allograft rejection is more frequent and often more severe; and there is no viable alternative if the graft fails. The recent ISHLT guidelines for the care of heart transplants included proposals for the use of rATG but highlighted that these were based largely on expert consensus [4]. The guidelines noted that universal administration of induction therapy in heart transplant recipients does not improve outcomes. Instead, the recommendations advise that rATG may be beneficial in patients at high risk for acute rejection and that polyclonal antibody preparations in general can support delaying calcineurin inhibitor (CNI) introduction in cases where there is a high risk of renal dysfunction [4].
Several recent developments have a potential impact on the decision whether and how to use rATG induction. First, rATG dosing has declined over the last decade [5], reducing dose-dependent complications. Second, donor heart allocation schemes in some countries now preferentially assign grafts to high-urgency cases. In the US, the proportion of recipients with the highest medical urgency status (1A) has increased fourfold in the last decade [1]. In Germany, changes to the allocation system mean that in practice only high-urgency hospitalized patients now receive a graft. These patients are in poor health or frequently on MCS and often have renal dysfunction, with a higher risk of infections at the time of transplant [6]. Thirdly, a widening choice of immunosuppressive therapies means that rATG is increasingly used as part of protocols to minimize exposure to CNIs or steroids in the early post-transplant period. Lastly, awareness of the prognostic importance of donor-specific antibodies (DSA), largely based on data in kidney transplantation [7,8] is influencing initial immunosuppressive protocols according to patients' pretransplant DSA status.
In the absence of evidence-based prescribing criteria, experts from Germany, Austria, and Switzerland convened to consider (i) the types of patients for whom rATG is a suitable option, and (ii) appropriate early maintenance immunosuppression in these cases, based on the available data and their own clinical experience.
Efficacy of rATG induction in heart transplantation
Heart transplant patients at high risk of acute rejection show a reduced risk of death secondary to graft rejection if lymphocyte-depleting induction is administered [9]. No randomized trial, however, has compared rATG induction specifically versus controls. Three randomized trials have assessed interleukin-2 receptor antagonist (IL-2RA) induction versus no induction [10–12], two of which observed a significant reduction in risk of rejection [11,12]. No survival benefit was detected. Two randomized trials have compared rATG versus IL-2RA induction in heart transplants at standard immunological risk. One of these demonstrated a lower rate of biopsy-proven acute rejection (BPAR) of grade 3 or 4 with rATG [13], while the second trial showed no significant difference between induction therapies based on BPAR grade >1B [14]. Retrospective studies (all with fewer than 50 patients) have observed a lower incidence or severity of acute rejection using rATG versus IL-2RA induction in standard-risk heart transplant populations [15–17]. In kidney transplantation, a much larger randomized trial (n = 278) of high-risk patients found that a 5-day course of rATG (cumulative dose 7.5 mg/kg) achieved a significantly lower rate of acute rejection and treated rejection in rATG-treated patients compared with the group given IL-2RA induction, although graft survival was unaffected [18].
Although the evidence base remains sparse, rATG induction in heart transplant patients at high risk of rejection (e.g., presensitized patients, younger patients, African American recipients) may be beneficial, as noted by the ISHLT [4].
Impaired renal function
The challenge of kidney dysfunction
Over half of all heart transplant recipients have chronic renal disease stage 3A or worse prior to surgery [19,20]. Following heart transplantation, kidney output frequently improves in response to improved hemodynamics. Some patients exhibit a markedly higher estimated GFR (eGFR) at hospital discharge versus pretransplant, followed by a decline over the first year post-transplant and a slower rate of deterioration thereafter [19,21]. A quarter of all patients, however, develop acute renal failure after heart transplantation [22]. Pre-existing chronic renal insufficiency and the use of cardiopulmonary bypass during surgery increase the risk of acute renal failure, which negatively affects short-term survival [22]. By 10 years, chronic kidney disease stage 3A or worse is almost universal [19] and approximately 10% of heart transplant patients eventually progress to chronic renal failure [23]. As would be expected, patients with poor kidney function pretransplant are significantly more likely to show deteriorating renal function after heart transplantation [20,21,24]. Other risk factors include older age [21,25] and possibly the presence of diabetes or hyperglycemia [20,21].
When planning the immunosuppressive regimen, it is important to identify recipients with predominantly hemodynamically mediated renal dysfunction in whom function is likely to resolve spontaneously post-transplant in response to adequate renal blood flow. Classifying patients according to the type of cardiorenal syndrome [26] can be helpful. Type 1 or 2 cardiorenal syndrome can be expected to show at least acceptable renal recovery after transplantation, and in these individuals, CNI minimization is less of a priority. In patients with Type 3 or 4 cardiorenal syndrome, long-term renal deterioration is largely inevitable and renoprotective strategies are a priority.
Renal histopathology after heart transplantation is complex to interpret [27], but progressive functional decline is partly due to chronic CNI exposure [23,28,29]. Early renal insufficiency caused by direct CNI-mediated renal arteriolar vasoconstriction can also occur [30]. In patients who have impaired renal function at the time of transplantation, reduced CNI exposure is associated with significant benefit for renal function [31], and renoprotective strategies center on CNI minimization, particularly delayed introduction to avoid the nephrotoxic effects of CNI therapy for as long as possible in the early post-transplant period.
rATG with delayed CNI introduction
Delaying CNI initiation is a widely used technique to protect the graft in the first few days after transplantation, when CNI exposure is conventionally highest, allowing the kidney to recover. rATG induction can provide immunosuppressive cover until CNI therapy is introduced. Retrospective studies of heart transplant patients receiving rATG have reported varying times for delay of CNI introduction [16,32–34], ranging from 3 days [16] to as long as 18 or 20 days [33,34]. In 2004, Cantarovich and colleagues published retrospective data demonstrating that rATG induction with delayed CNI (started only when serum creatinine fell below 150 μmol/l) provided comparable efficacy to rATG with immediate CNI [33]. A case-control study by Delgado et al. [16] reported outcomes in seven patients given rATG induction with cyclosporine (CsA) delayed until a mean of 3.2 days post-transplant and in seven patients who received basiliximab induction with CsA started at a mean of 7.3 days. All patients had pre-operative renal dysfunction. Patients in both groups showed improved renal function after transplantation, with slightly better function in the rATG group (significant at month 30). Cellular rejection was less frequent in the rATG arm [16].
The optimal timing for CNI initiation in patients receiving rATG induction has not been established. It is usual, however, to start CNI during days 4–7, or when eGFR and urinary output increases. Some centers monitor the T-cell or CD3+ lymphocyte count to determine when to initiate CNI. Comparative studies of different rATG induction regimens in heart transplantation would be very helpful.
rATG with reduced-exposure CNI
There is good evidence that rATG induction with reduced-exposure CNI, a mammalian target of rapamycin (mTOR) inhibitor, mycophenolate mofetil (MMF), and steroids provides similar efficacy to a conventional CNI regimen [35,36]. The recent SCHEDULE study compared low-exposure CsA with everolimus (ERL) versus standard-exposure CsA, both with MMF and steroids, in de novo heart transplant patients [35]. All patients received rATG induction. At 7–11 weeks post-transplant, CsA was withdrawn in the ERL arm and ERL exposure was increased. By month 12, there was a clear improvement in renal function in the reduced-exposure CNI group (mean eGFR 79.8 vs. 61.5 ml/min/1.73 m2, P < 0.001). During the period up to CNI withdrawal, the rates of any BPAR, BPAR ≥2R or treated rejection were similar in both groups. After CNI withdrawal, however, BPAR became more frequent in the CNI-free arm. A randomized trial of maintenance heart transplant patients with mild-to-moderate renal insufficiency who continued standard CNI therapy or switched to sirolimus observed that low MMF dose (≤1000 mg/day) and nonwhite race were independent predictors for risk of rejection after switch to mTOR inhibition [37].
Another randomized trial (A2310) compared reduced-exposure CsA with ERL versus standard-exposure CsA with MMF, both with steroids, in de novo heart transplant patients [36]. Approximately 30% of patients received rATG induction. The incidence of BPAR grade ≥3A to month 12 was similar in both groups. However, rATG-treated patients receiving reduced-CsA with ERL showed a higher rate of early (<3 months) infectious deaths, particularly in patients on a ventricular assist device (VAD) prior to transplant [36], suggesting overimmunosuppression. Lower initial CNI targets than the A2310 study in patients receiving rATG induction appear preferable if rATG is used, particularly in VAD patients, when given with ERL or MMF plus steroids. The CsA target in the A2310 trial was 200–350 ng/ml during month 1, but there are no data to indicate what lower level of exposure may be appropriate. The initial CNI target should certainly be reduced considerably in heart transplant patients if rATG is given and both ERL and MMF are given concomitantly, based on results of the SCHEDULE study, for example, CsA 75–175 ng/ml. There are no data to indicate an appropriate tacrolimus (TAC) target range with concomitant ERL in heart transplantation; some centers have used ranges of 5–8 ng/ml or even 3–5 ng/ml, but no recommendations can be made. ERL, if used, should be maintained indefinitely in the range 3–8 ng/ml and if MMF is given the dose remains at 2 g/day. After month 6, steroid doses can be reduced and steroid withdrawal may be feasible after 12–18 months.
rATG with CNI avoidance
Delayed and/or reduced CNI exposure can only partly counteract the chronic nephrotoxic effect of CNI therapy in the long-term. However, rATG induction with complete CNI avoidance does not appear to offer adequate immunosuppressive efficacy in de novo heart transplant patients. Data with rATG are lacking, but in a pilot trial Meiser et al. [38] examined CNI avoidance in eight patients using a regimen of sirolimus, MMF, and corticosteroids, with ATG-Fresenius induction. The acute rejection rate was 25% during 3- to 12-month follow-up, and renal function improved then remained stable throughout follow-up. However, a subsequent retrospective analysis showed that among 15 patients receiving this regimen at the same center, there was numerically more acute rejection than in patients given CNI therapy, and there were more frequent discontinuations from the CNI-free regimen due to intolerance [39]. Confirmatory data are lacking but the frequency of switch to CNI therapy and high rates of sirolimus-related side effects [38,39]—and the need for a very high biopsy rate to monitor for subclinical rejection—mean that this is unlikely to be a successful protocol. It cannot provide a safe and stable patient course in the sensitive early period after heart transplantation.
Patient selection based on renal function
Renal dysfunction pre- or peri-operatively is a relatively well-established indication for rATG induction with delayed CNI administration for 4–5 days or based on renal recovery (Fig.1a). As eGFR has only limited accuracy in this setting, it is advisable to measure 24-h urine output prior to transplantation, with estimated GFR and protein:creatinine ratio for supplementary information. A suitable threshold may be eGFR <40 ml/min/1.73 m2, although some centers apply a higher cut-off (e.g., 60 ml/min/1.73 m2) and some centers also stipulate a protein/creatinine ratio ≥0.3 when defining renal dysfunction, although it should be noted that neither eGFR nor proteinuria correlate with renal fibrosis on biopsy [40].
Figure 1.
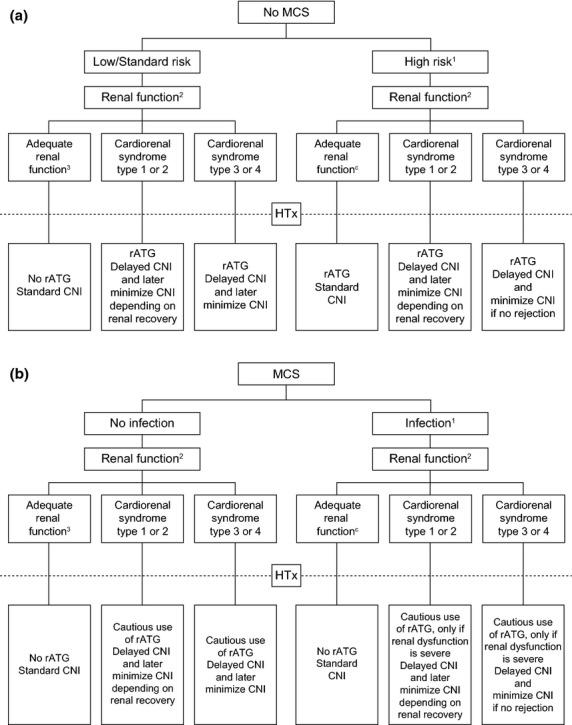
Suggested algorithm for use of rATG induction in heart transplant patients (a) without mechanical circulatory support (MCS) or (b) with MCS. CNI, calcineurin inhibitor; HTx, heart transplantation; rATG, rabbit antithymocyte globulin. (a) 1High immunological risk (e.g. pre-transplant DSA, >4 HLA mismatches, black); post-puertum females; older age (>60–65 years); younger age (e.g. <35 years); children (e.g. <10 years); postoperative bleeding; history of malignancy. 224-h urine output, estimated GFR, protein/creatinine ratio; define cause of renal dysfunction. 3Estimated GFR ≥60 ml/min/1.73 m2 and protein:creatinine ≤0.3 in 24-h urine output analysis. (b) 1Driveline orificium; mediastinitis; positive blood culture; temperature >38.5 °C (F). 224-h urine output, estimated GFR protein/creatinine ratio; define cause of renal dysfunction. 3Estimated GFR ≥60 ml/min/1.73 m2 and protein:creatinine ≤0.3 in 24-h urine output analysis.
Renal dysfunction is particularly frequent in recipients on a VAD or an intra-aortic balloon pump pretransplant, who are also at increased risk of acute renal failure post-transplant [41]. In this high-risk group, rATG with delayed CNI may maximize early renal function but the situation is complicated by the high risk of infection in patients on VAD. Extreme caution is warranted in light of data from the A2310 data showing increased early deaths from infectious causes in VAD patients. IL-2RA induction, or low-dose rATG with low-exposure CNI and an mTOR inhibitor and steroids may be a suitable option in VAD patients with severe pretransplant renal insufficiency, but this cannot be supported by evidence. It is important to note that mTOR inhibitor therapy is not recommended in patients on MCS until after 4 weeks post-transplant due to the risk of wound healing complications [42].
If acute renal failure develops immediately post-transplant, introduction of rATG induction with delayed CNI or switch to a reduced-CNI strategy could be an appropriate option, but again this is unsupported by data. Alternatively, this situation could prompt CNI reduction with introduction of an mTOR inhibitor or MMF and no induction. Trials to determine the optimal management in this situation are required.
Certain caveats must be considered. Patients with renal dysfunction who are at high risk for rejection (e.g., highly sensitized patients) are not suitable candidates for CNI delay or reduction even with rATG induction. De novo immunosuppression with rATG induction, reduced-exposure CNI and an mTOR inhibitor is not advisable if proteinuria is >0.5 g/day at the time of transplant. Later switch to a CNI minimization regimen should not be considered if the patient has experienced early acute cellular rejection (grade ≥IIR [43]) or any antibody-mediated rejection (AMR). Moreover, rATG induction with CNI delay or minimization requires close monitoring of maintenance drug concentrations and regular biopsies. Thus, patients who are geographically remote may be less suitable. If a patient proves to be poorly compliant, low-exposure CNI targets may need to be revised upwards to reduce the risk of break-through rejection. Conversely, older recipients with a lower risk of rejection may do well on a reduced-CNI regimen and are attractive candidates for renal-sparing regimens because age is a risk factor for renal failure after heart transplantation.
Steroid minimization
In kidney transplantation, rATG induction with early withdrawal of steroids (days 7–8 post-transplant) achieves similar rejection rates to a standard steroid regimen [44,45]. In heart transplantation, however, the potentially fatal consequences mean a more cautious approach to aggressive steroid minimization. A small trial randomized 32 low-risk heart transplant recipients to rATG induction with no steroids or to no induction with standard steroids [46]. All patients received TAC at a relatively high exposure (15–20 ng/ml to month 3) with MMF. The incidence of acute cellular rejection was similar in both arms, but the high CNI exposure is a potential cause for concern. In children, where the imperative for steroid minimization is greatest, a retrospective analysis of 70 patients (six of whom were sensitized) assessed outcomes after rATG induction and a single intravenous dose of methylprednisolone but no oral steroids, combined with TAC and MMF [47]. Steroids were introduced as maintenance therapy in 16% of patients. The incidence of rejection at 6 months was 8%. This encouraging result has not been confirmed by other centers although there are sporadic reports of steroid-free maintenance in children treated with rATG induction [48]. Overall, the data on rATG induction with steroid-free immunosuppression or very early steroid withdrawal after heart transplantation are too sketchy to draw any firm conclusions.
Patients on mechanical circulatory support
Death from infection is a major cause of mortality after heart transplantation, particularly in the first year [2] (Fig.1b). Patients on MCS are at particularly high risk of a bloodstream infection [6] and infectious death [48], a growing problem as the use of VAD continues to increase [1]. This presents a clinical dilemma because these patients are more sensitized [49] and at increased immunological risk [50], exhibiting raised levels of cytokines [51], B-cells and immunoglobulins [52]. Rejection is more severe in VAD patients [53].
In patients on VAD who have an ongoing infection, IL-2RA induction with delayed CNI [16,32] or, preferably, reduced-exposure CNI [36,54] may be feasible. However, it should be noted that the only available IL-2RA agent, basiliximab, is licensed exclusively for use in kidney transplantation. Recently, a warning about off-label use of IL2-RA induction after heart transplantation was distributed in Germany by the manufacturer in agreement with the European Medicines Agency (EMA) because of an increased risk of cardiac events (e.g., arrhythmia) in heart transplant recipients compared to other induction therapies. If renal dysfunction is severe, a short/low dose of rATG could be considered with reduced-exposure CNI, but in the presence of infection minimizing the risk of infectious death is likely to be the paramount clinical concern. Even if there is no infection at the time of transplant, rATG may be inadvisable to minimize the risk of post-transplant infection [36] and for VAD patients with adequate renal function rATG should not be given. Some centers entirely avoid rATG induction in VAD patients. No comparative studies of different immunosuppressive regimens have been carried out specifically in patients on VAD prior to transplant, but results from the subpopulation of VAD patients in the A2301 study [36] have raised concerns about infectious deaths if rATG induction is administered with standard early maintenance immunosuppression in this group of patients.
Presensitized patients
Heart transplant patients are at particular risk of sensitization prior to transplant. In addition to standard risk factors in solid organ recipients such as blood transfusion [55], pregnancy [56], cryopreserved allograft tissue [57], and retransplantation [58], many have become sensitized by previous cardiac surgery or by use of a MCS device [49,59]. Children, in particular, are often presensitized from use of allograft material during previous reconstructive cardiac surgery. Additionally, heart grafts are less well-matched than for kidney transplantation, as the pool of organs is smaller, transplantation is more urgent, and graft storage times are shorter. Recipients with pretransplant DSA are far more likely to experience antibody-mediated or cellular rejection [60] and graft failure [61] despite desensitization measures [62,63], which tend to be applied more aggressively than in other types of solid organ transplantation because it is often not possible to await the results of cross-match testing.
A consensus conference in 2009 recommended that rATG induction should be considered for presensitized heart transplant patients due to the risk of AMR, with TAC, MMF, and steroids as maintenance immunosuppression [62]. The rationale for use of rATG is based on its multiple cellular targets: rATG targets plasma cells in addition to peripheral T-lymphocytes, B-lymphocytes and natural killer (NK) cells [64,65]. Surface antigens on plasma cells are believed to play a key role in the onset of AMR. Data in heart transplantation are lacking, however, regarding the effect of rATG induction on the rate of AMR and on development of de novo DSA. In kidney transplantation, a prospective nonrandomized study of 114 moderately sensitized patients receiving rATG or IL-2RA induction reported a significantly lower rate of de novo DSA and AMR in the rATG-treated patients [66]. Multivariate analysis showed that induction with rATG was the single most important variable associated with either event.
On a practical note, it should be borne in mind that if rATG is in the bloodstream when plasmapheresis is carried out in presensitized patients, it will be filtered out and rATG levels are likely to become subtherapeutic unless another dose is given. To help minimize this effect, rATG infusion can be carried out at the end of a plasmapheresis session, allowing a 24–48 h window before the next session. Also, in this high-risk group, initial maintenance therapy with TAC and MMF may help to avoid the need for subsequent regimen switches, which can trigger rejection.
Evidence relating to rATG and the risk of DSA production and risk of AMR in heart transplant patients is awaited with interest and may lead to the use of rATG induction in presensitized individuals. Specifically, prospective studies with protocol-specified DSA monitoring to establish the effect of rATG induction versus no induction or IL-2RA induction are required to assess DSA recurrence after desensitization, rates of AMR in presensitized or otherwise high-risk individuals, and development of de novo DSA. More generally, the challenge remains to identify accurate criteria to define ‘high risk’ for de novo DSA or for AMR other than pretransplant DSA. Previous pregnancy is one relatively well-established risk factor [55] and, particularly for multiple pregnancies, may necessitate more intensive induction following heart transplantation.
Risk of malignancy
Experience from the 1980s to the mid-1990s showed a significantly increased risk of lymphoma with rATG induction [67,68] unless antiviral prophylaxis was given [68]. Since, both rATG dose levels [5] and the overall burden of immunosuppression has declined, particularly lower CNI exposure and lower steroid doses [69]. More recently, an analysis of ISHLT data based on 3,895 heart transplants during 1995–1997 found no association between rATG use and risk of malignancy [69]. Consistent with this, a systematic review of studies published during 1999–2009 found a low rate of post-transplant proliferative disorder (PTLD) at a median follow-up of 5 years after heart transplantation in rATG-treated patients (1.05%) [70]. The authors found no evidence that use of rATG was associated with PTLD, although there was a nonsignificant trend to more PTLD when rATG dose was 7.5 mg/kg or higher (1.55% vs. 0.50% with lower doses, P = 0.18) and the rate of PTLD was highest (2.62%) in heart transplant patients given rATG ≥7.5 mg/kg with no antiviral prophylaxis [70]. Antiviral prophylactic therapy is now generally routine for the first 3 months post-transplant.
Pediatric recipients, particularly those aged <10 years, are at vastly increased risk of lymphoma compared to older patients [67]. There is evidence from pediatric heart transplant populations that a higher cumulative dose of rATG increases risk of PTLD [71] and that rATG induction per se is a risk factor for increased Epstein Barr virus load [72], although the literature also includes reports of low rates of lymphoma in rATG-treated children given relatively high doses [73,74]. In view of the very high rate of lymphoma in children undergoing heart transplantation, and the apparent increase in risk with high-dose rATG [70,71], a maximum cumulative rATG dose of 3–4.5 mg/kg may be appropriate, but this is unconfirmed by trial data.
In summary, the risk for developing lymphoma or other neoplasias after rATG induction has not been elevated in recent studies using an adequate dosage. An elevated incidence of cancer was reported in earlier studies with extremely high rATG doses. In patients with a history of malignancy more than 1 year prior to heart transplantation, there is no evidence to contraindicate rATG administration.
rATG dosing
The rATG license recommends a dose of 1.5 mg/kg/day for 7–14 days in kidney transplants. In fact, rATG dosing has declined successively over the last decade and no longer reflects the license [5]. In kidney transplantation, a cumulative dose of 6 mg/kg achieves similar efficacy to higher doses [75] and is generally considered appropriate [65,76]; lower doses may be suitable in patients at low immunological risk such as living-donor [77] or elderly patients [78]. As rATG is used off-label outside kidney transplantation, there is no dosing recommendation for heart transplant patients and there is almost a complete absence of comparative dosing studies in heart transplantation. One retrospective study, in which rATG 1.5 mg/kg/day was given for 7 days in 166 high-risk heart transplant patients and for 5 days in 87 lower-risk patients, reported similar rates of rejection and survival to 1 year and no increase in infection or malignancy with the longer dosing regimen [79], but other comparative analyses of different doses are lacking.
Currently, rATG dosing in heart transplantation is largely empiric and there is a wide variation in protocols. Generally, doses are lower than in the past, with few centers administering total doses >7.5 mg/kg and some centers giving only a single dose of 1 mg/kg/day or a cumulative dose of 3.5 mg/kg in total. Studies of outcomes associated with different dosing strategies are urgently required.
Similarly, there is no consensus on the use of intra-operative administration prior to clamp opening. One factor to take into account is the dose-dependent thrombocytopenic effect of rATG [5]. Mild thrombocytopenia has been reported in children receiving intra- and peri-operative rATG [73]. Where used, the dose should take into account the risk of thrombocytopenia induced by the intra-operative pump, and the fact that VAD patients are prone to postsurgical bleeding. An alternative strategy is to wait up to 4–6 h after closure to see if bleeding occurs and postpone rATG if necessary.
The duration of rATG infusions is now often extended, in some centers to as long as 24 h, to avoid infusion-related adverse events such as a rise in temperature, but the effect of different timings has not been assessed.
Conclusions
When assessing rATG induction in heart transplantation, the most striking feature is the shortage of data from controlled trials, as reviewed recently [80]. This compels the clinician to base prescribing on suboptimal study data and clinical experience.
We have suggested a proposed algorithm for rATG induction decision-making (Fig.1), but fully recognize that in many areas this cannot be supported by clinical evidence. Furthermore, we have also suggested an overview of rATG induction and early maintenance regimens in particular categories of heart transplantation (Table1). These are derived from our own experience where data are lacking—experience that is biased toward organ allocation to high-urgency cases and in which immunosuppressive intensity is generally lower than some other parts of the world, notably the US. Finally, we have recommended priorities for future studies of rATG induction, focusing on those which we consider to be the most urgent (Table2).
Table 1.
Suggested strategy for rATG induction according to characteristics of heart transplant patients
| Characteristic | Category/comment | Suggested strategy |
|---|---|---|
| Renal dysfunction*,† | Cardiorenal Type 1 or 2 (no structural damage to kidneys) | rATG induction with delayed CNI, consider CNI minimization depending on renal recovery |
| Cardiorenal Type 3 or 4 (structural damage to kidneys, for example, diabetic nephropathy) | rATG induction with delayed CNI and CNI minimization | |
| Acute renal failure (e.g., due to surgical trauma) | rATG induction with delayed CNI, consider CNI minimization depending on renal recovery | |
| High immunological risk (e.g., pretransplant DSA, >4 HLA mismatches, black) | High risk of rejection | Reduced risk of rejection with rATG induction Tacrolimus + MMF as initial immunosuppression may reduce need for switch |
| Post-puertum females | Can be highly sensitized | Strong candidates for rATG induction |
| Older age (>60–65 years) | Tend to have impaired renal function Lower risk of rejection May be at increased risk of infectious death |
Good candidates for lower-dose rATG induction with low CNI |
| Younger age (e.g., 10 to <35 years) | Increased risk of rejection versus older recipients | More likely to benefit from rATG induction to reduce risk of rejection than older recipients |
| Children (e.g., <10 years) | Markedly increased risk of PTLD/lymphoma | Avoid overimmunosuppression Consider low-dose rATG with low-dose CNI |
| VAD | No renal dysfunction | Unlikely to require rATG if VAD (concern over risk of infectious death) |
| Renal dysfunction | As per ‘renal dysfunction’ above if severe renal insufficiency is present (concern over infectious death) | |
| Ongoing infection | No induction due to high risk of infectious death or, if renal dysfunction is severe, consider lower/shorter rATG induction with decreased maintenance immunosuppression | |
| Postoperative bleeding | Particularly likely in VAD patients Dose-dependent risk of thrombocytopenia with rATG therapy |
Consider delaying rATG for 4–6 h postoperatively to check if bleeding occurs If rATG is given during bleeding, also administer thrombocytes and repeat after every dose of rATG |
| History of malignancy | No evidence for increased risk or recurrence with rATG induction | rATG induction if indicated by risk status or renal function, with CNI minimization |
CNI, calcineurin inhibitor; DSA, donor-specific antibodies; HLA, human leukocyte antigen; MMF, mycophenolate mofetil; PTLD, post-transplant lymphoproliferative disorder; rATG, rabbit antithymocyte globulin; VAD, ventricular assist device.
CNI minimization should be avoided or undertaken cautiously in patients who have early acute rejection (grade > IIR) or any antibody-mediated rejection, are noncompliant, geographically remote due to difficulties in follow-up, and necessitates regular protocol biopsies. Patients with proteinuria >0.5 g/day may be unsuitable for mTOR inhibitor therapy.
Assess estimated GFR (e.g., abbreviated MDRD formula) and urine output, and identify cause, for example, diabetic nephropathy, chronic congestive heart failure.
Threshold for ‘renal dysfunction’ has not been established, for example, 40–60 ml/min/1.73 m2.
Table 2.
Suggested priorities for future studies of rATG induction in heart transplantation
| Comparison | Heart transplant population | Key endpoints |
|---|---|---|
| rATG versus no induction | Sensitized patients | Rejection rate, de novo DSA development, AMR |
| rATG + delayed CNI (day 7) for 7 days versus IL-2RA induction or no induction | Patients with renal impairment | Rejection rate, renal function, need for dialysis early after TX |
| rATG + low-exposure CNI versus no induction + standard-exposure CNI | Standard cohort | Rejection, renal function, side effects, infections |
AMR, antibody-mediated rejection; CNI, calcineurin inhibitor; DSA, donor-specific antibodies; rATG, rabbit antithymocyte globulin.
The evidence base for rATG induction in heart transplantation is far less extensive than for other immunosuppressive agents, or indeed for rATG in other indications. Nevertheless, it is a valuable component of the immunosuppressive armamentarium, particularly for reducing rejection in high-risk individuals and supporting the delay or early minimization of CNI exposure to restore renal function. Further trials should be undertaken to refine the largely empiric approach which currently governs its use in the heart transplant population.
Funding
The authors received travel funding from Sanofi to attend a meeting at which the data for inclusion in the paper were discussed and presentations made by the authors.
References
- 1. Organ Procurement and Transplant Network (OPTN) Annual Report 2012 http://srtr.transplant.hrsa.gov/annual_reports/2012 Accessed 11 June 2014.
- 2.Lund LH, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Official Adult Heart Transplant Report–2013; focus theme: age. J Heart Lung Transplant. 2013;32:951. doi: 10.1016/j.healun.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Dipchand AI, Kirk R, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Sixteenth Official Pediatric Heart Transplantation Report – 2013; focus theme: age. J Heart Lung Transplant. 2013;32:979. doi: 10.1016/j.healun.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Costanzo MR, Dipchand A, Starling R, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29:914. doi: 10.1016/j.healun.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 5.Gaber AO, Monaco AP, Russell JA, Lebranchu Y, Mohty M. Rabbit antithymocyte globulin (Thymoglobulin): 25 years and new frontiers in solid organ transplantation and haematology. Drugs. 2010;70:691. doi: 10.2165/11315940-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Toda K, Yonemoto Y, Fujita T, et al. Risk analysis of bloodstream infection during long-term left ventricular assist device support. Ann Thorac Surg. 2012;94:1187. doi: 10.1016/j.athoracsur.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Loupy A, Lefaucheur C, Vernerey D, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med. 2013;369:1215. doi: 10.1056/NEJMoa1302506. [DOI] [PubMed] [Google Scholar]
- 8.Lionaki S, Panagiotellis K, Iniotaki A, Boletis JN. Incidence and clinical significance of de novo donor specific antibodies after kidney transplantation. Clin Dev Immunol. 2013;2013:849835. doi: 10.1155/2013/849835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins R, Kirklin JK, Brown RN, et al. To induce or not to induce: do patients at greatest risk for fatal rejection benefit from cytolytic induction therapy? J Heart Lung Transplant. 2005;24:392. doi: 10.1016/j.healun.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Mehra MR, Zucker MJ, Wagoner L, et al. A multicenter, prospective, randomized, double-blind trial of basiliximab in heart transplantation. J Heart Lung Transplant. 2005;24:1297. doi: 10.1016/j.healun.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Beniaminovitz A, Itescu S, Lietz K, et al. Prevention of rejection in cardiac transplantation by blockade of the interleukin-2 receptor with a monoclonal antibody. N Engl J Med. 2000;342:613. doi: 10.1056/NEJM200003023420902. [DOI] [PubMed] [Google Scholar]
- 12.Hershberger RE, Starling RC, Eisen HJ, et al. Daclizumab to prevent rejection after cardiac transplantation. N Engl J Med. 2005;352:2705. doi: 10.1056/NEJMoa032953. [DOI] [PubMed] [Google Scholar]
- 13.Carrier M, Leblanc MH, Perrault LP, et al. Basiliximab and rabbit anti-thymocyte globulin for prophylaxis of acute rejection after heart transplantation: a non-inferiority trial. J Heart Lung Transplant. 2007;26:258. doi: 10.1016/j.healun.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Mattei M, Redonnet M, Gandjbakhch I, et al. Lower risk of infectious deaths in cardiac transplant patients receiving basiliximab versus anti-thymocyte globulin as induction therapy. J Heart Lung Transplant. 2007;26:693. doi: 10.1016/j.healun.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Carlsen J, Johansen M, Boesgaard S, et al. Induction therapy after cardiac transplantation: a comparison of anti-thymocyte globulin and daclizumab in the prevention of acute rejection. J Heart Lung Transplant. 2005;24:296. doi: 10.1016/j.healun.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Delgado DH, Miriuka SG, Cusimano RJ, Feindel C, Rao V, Ross HJ. Use of basiliximab and cyclosporine in heart transplant patients with pre-operative renal dysfunction. J Heart Lung Transplant. 2005;24:166. doi: 10.1016/j.healun.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 17.Flaman F, Zieroth S, Rao V, Ross H, Delgado DH. Basiliximab versus rabbit anti-thymocyte globulin for induction therapy in patients after heart transplantation. J Heart Lung Transplant. 2006;25:1358. doi: 10.1016/j.healun.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Brennan DC, Daller JA, Lake KD, et al. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355:1967. doi: 10.1056/NEJMoa060068. [DOI] [PubMed] [Google Scholar]
- 19.Hamour IM, Omar F, Lyster HS, Palmer A, Banner NR. Chronic kidney disease after heart transplantation. Nephrol Dial Transplant. 2009;24:1655. doi: 10.1093/ndt/gfn759. [DOI] [PubMed] [Google Scholar]
- 20.Navarro-Manchón J, Martínez-Dolz L, Almenar Bonet L, et al. Predictors of renal dysfunction at 1 year in heart transplant patients. Transplantation. 2010;89:977. doi: 10.1097/tp.0b013e3181cbe024. [DOI] [PubMed] [Google Scholar]
- 21.Lachance K, White M, Carrier M, et al. Long-term evolution, secular trends, and risk factors of renal dysfunction following cardiac transplantation. Transpl Int. 2014;27:824. doi: 10.1111/tri.12340. doi: 10.1111/tri.12340. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Gude E, Andreassen AK, Arora S, et al. Acute renal failure early after heart transplantation: risk factors and clinical consequences. Clin Transplant. 2010;24:E207. doi: 10.1111/j.1399-0012.2010.01225.x. [DOI] [PubMed] [Google Scholar]
- 23.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 24.Hertz MI. The Registry of the International Society for Heart and Lung Transplantation-Introduction to the 2012 annual reports: new leadership, same vision. J Heart Lung Transplant. 2012;31:1045. doi: 10.1016/j.healun.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Garrido IP, Crespo-Leiro MG, Paniagua MJ, et al. Independent predictors of renal dysfunction after heart transplantation in patients with normal pretransplant renal function. J Heart Lung Transplant. 2005;24:1226. doi: 10.1016/j.healun.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 26.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 27.Pinney SP, Balakrishnan R, Dikman S, et al. Histopathology of renal failure after heart transplantation: a diverse spectrum. J Heart Lung Transplant. 2012;31:233. doi: 10.1016/j.healun.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Bloom RD, Doyle AM. Kidney disease after heart and lung transplantation. Am J Transplant. 2006;6:671. doi: 10.1111/j.1600-6143.2006.01248.x. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein DJ, Zuech N, Sehgal V, Weinberg AD, Drusin R, Cohen D. Cyclosporine-associated end-stage nephropathy after cardiac transplantation: incidence and progression. Transplantation. 1997;63:664. doi: 10.1097/00007890-199703150-00009. [DOI] [PubMed] [Google Scholar]
- 30.Baran DA, Galin ID, Gass AL. Calcineurin inhibitor-associated early renal insufficiency in cardiac transplant recipients: risk factors and strategies for prevention and treatment. Am J Cardiovasc Drugs. 2004;4:21. doi: 10.2165/00129784-200404010-00003. [DOI] [PubMed] [Google Scholar]
- 31.Cornu C, Dufays C, Gaillard S, et al. Impact of the reduction of calcineurin inhibitors on renal function in heart transplant patients: a systematic review and meta-analysis. Br J Clin Pharmacol. 2014;78:24. doi: 10.1111/bcp.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberg PB, Vriesendorp AE, Drazner MH, et al. Induction therapy with basiliximab allows delayed initiation of cyclosporine and preserves renal function after cardiac transplantation. J Heart Lung Transplant. 2005;24:1327. doi: 10.1016/j.healun.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Cantarovich M, Giannetti N, Barkun J, Cecere R. Antithymocyte globulin induction allows a prolonged delay in the initiation of cyclosporine in heart transplant patients with postoperative renal dysfunction. Transplantation. 2004;78:779. doi: 10.1097/01.tp.0000130179.18176.3d. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez-Lazaro IJ, Almenar-Bonet L, Martinez-Dolz L, et al. Repeated daclizumab administration to delay the introduction of calcineurin inhibitors in heart transplant patients with postoperative renal dysfunction. Rev Esp Cardiol. 2011;64:237. doi: 10.1016/j.recesp.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Andreassen AK, Andersson B, Gustafsson F, et al. Everolimus initiation and early calcineurin inhibitor withdrawal in heart transplant recipients: a randomized trial. Am J Transplant. 2014;14:1828. doi: 10.1111/ajt.12809. [DOI] [PubMed] [Google Scholar]
- 36.Eisen HJ, Kobashigawa J, Starling RC, et al. Everolimus versus mycophenolate mofetil in heart transplantation: a randomized, multicenter trial. Am J Transplant. 2013;13:1203. doi: 10.1111/ajt.12181. [DOI] [PubMed] [Google Scholar]
- 37.Zuckermann A, Eisen H, Tai SS, Li H, Hahn C, Crespo-Leiro MG. Sirolimus conversion after heart transplant: risk factors for acute rejection and predictors of renal function response. Am J Transplant. 2014;14:2048. doi: 10.1111/ajt.12833. [DOI] [PubMed] [Google Scholar]
- 38.Meiser B, Reichart B, Adamidis I, Uberfuhr P, Kaczmarek I. First experience with de novo calcineurin-inhibitor-free immunosuppression following cardiac transplantation. Am J Transplant. 2005;5(4 Pt 1):827. doi: 10.1111/j.1600-6143.2005.00757.x. [DOI] [PubMed] [Google Scholar]
- 39.Kaczmarek I, Zaruba MM, Beiras-Fernandez A, et al. Tacrolimus with mycophenolate mofetil or sirolimus compared with calcineurin inhibitor-free immunosuppression (sirolimus/mycophenolate mofetil) after heart transplantation: 5-year results. J Heart Lung Transplant. 2013;32:277. doi: 10.1016/j.healun.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 40.Labban B, Arora N, Restaino S, Markowitz G, Valeri A, Radhakrishnan J. The role of kidney biopsy in heart transplant candidates with kidney disease. Transplantation. 2010;89:887. doi: 10.1097/TP.0b013e3181cd4abb. [DOI] [PubMed] [Google Scholar]
- 41.Alba AC, Rao V, Ivanov J, Ross HJ, Delgado DH. Predictors of acute renal dysfunction after ventricular assist device placement. J Card Fail. 2009;15:874. doi: 10.1016/j.cardfail.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 42.Zuckermann A, Barten MJ. Surgical wound complications after heart transplantation. Transpl Int. 2011;24:627. doi: 10.1111/j.1432-2277.2011.01247.x. [DOI] [PubMed] [Google Scholar]
- 43.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 44.Woodle ES, First MR, Pirsch J, et al. A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg. 2008;248:564. doi: 10.1097/SLA.0b013e318187d1da. [DOI] [PubMed] [Google Scholar]
- 45.Woodle ES, Peddi VR, Tomlanovich S, Mulgaonkar S, Kuo PC TRIMS Study Investigators. A prospective, randomized, multicenter study evaluating early corticosteroid withdrawal with Thymoglobulin in living-donor kidney transplantation. Clin Transplant. 2010;24:73. doi: 10.1111/j.1399-0012.2009.01127.x. [DOI] [PubMed] [Google Scholar]
- 46.Yamani MH, Taylor DO, Czerr J, et al. Thymoglobulin induction and steroid avoidance in cardiac transplantation: results of a prospective, randomized, controlled study. Clin Transplant. 2008;22:76. [PubMed] [Google Scholar]
- 47.Singh TP, Faber C, Blume ED, et al. Safety and early outcomes using a corticosteroids-avoidance immunosuppressive protocol in pediatric heart transplant recipients. J Heart Lung Transplant. 2010;29:517. doi: 10.1016/j.healun.2009.11.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parisi F, Danesi H, Squitieri C, Di Chiara L, Ravà L, Di Donato RM. Thymoglobuline use in pediatric heart transplantation. J Heart Lung Transplant. 2003;22:591. doi: 10.1016/s1053-2498(02)00813-6. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez-Stawinski GV, Cook DJ, Chang AS, et al. Ventricular assist devices and aggressive immunosuppression: looking beyond overall survival. J Heart Lung Transplant. 2006;25:613. doi: 10.1016/j.healun.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 50.Pamboukian SV, Costanzo MR, Dunlap S, et al. Relationship between bridging with ventricular assist device on rejection after heart transplantation. J Heart Lung Transplant. 2005;24:310. doi: 10.1016/j.healun.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 51.Birks EJ, Khaghani A, Bowles C, et al. Serum cytokines in left ventricular assist device candidates. Transplant Proc. 2001;33:1974. doi: 10.1016/s0041-1345(00)02759-7. [DOI] [PubMed] [Google Scholar]
- 52.Erren M, Schlüter B, Fobker M, et al. Immunologic effects of implantation of left ventricular assist devices. Transplant Proc. 2001;33:1965. doi: 10.1016/s0041-1345(00)02756-1. [DOI] [PubMed] [Google Scholar]
- 53.Malickaite R, Rucinskas K, Staneviciene A, et al. Sensitisation and post-transplant course after the implantation of ventricular assist device. Interact Cardiovasc Thorac Surg. 2009;8:339. doi: 10.1510/icvts.2008.192567. [DOI] [PubMed] [Google Scholar]
- 54.Cantarovich M, Ross H, Arizón JM, et al. Benefit of Neoral C2 monitoring in de novo cardiac transplant recipients receiving basiliximab induction. Transplantation. 2008;85:992. doi: 10.1097/TP.0b013e318169bf43. [DOI] [PubMed] [Google Scholar]
- 55.Scornik JC, Meier-Kriesche HU. Blood transfusions in organ transplant patients: mechanisms of sensitization and implications for prevention. Am J Transplant. 2011;11:1785. doi: 10.1111/j.1600-6143.2011.03705.x. [DOI] [PubMed] [Google Scholar]
- 56.Pollack MS, Trimarchi HM, Riley DJ, Casperson PR, Manyari LE, Suki WN. Shared cadaver donor-husband HLA class I mismatches as a risk factor for renal graft rejection in previously pregnant women. Hum Immunol. 1999;60:1150. doi: 10.1016/s0198-8859(99)00104-4. [DOI] [PubMed] [Google Scholar]
- 57.Hooper DK, Hawkins JA, Fuller TC, Profaizer T, Shaddy RE. Panel reactive antibodies late after allograft implantation in children. Ann Thorac Surg. 2005;79:641. doi: 10.1016/j.athoracsur.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 58.Conway J, Manlhiot C, Kirk R, Edwards LB, McCrindle BW, Dipchand AI. Mortality and morbidity after retransplantation after primary heart transplant in childhood: an analysis from the registry of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2014;33:241. doi: 10.1016/j.healun.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 59.Joyce DL, Southard RE, Torre-Amione G, Noon GP, Land GA, Loebe M. Impact of left ventricular assist device (LVAD)-mediated humoral sensitization on post-transplant outcomes. J Heart Lung Transplant. 2005;24:2054. doi: 10.1016/j.healun.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 60.Reinsmoen NL, Lai CH, Mirocha J, et al. Increased negative impact of donor HLA-specific together with non-HLA-specific antibodies on graft outcome. Transplantation. 2014;97:595. doi: 10.1097/01.TP.0000436927.08026.a8. [DOI] [PubMed] [Google Scholar]
- 61.Ho EK, Vlad G, Vasilescu ER, et al. Pre- and posttransplantation allosensitization in heart allograft recipients: major impact of de novo alloantibody production on allograft survival. Hum Immunol. 2011;72:5. doi: 10.1016/j.humimm.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 62.Eckman PM. Immunosuppression in the sensitized heart transplant recipient. Curr Opin Organ Transplant. 2010;15:650. doi: 10.1097/MOT.0b013e32833de9b2. [DOI] [PubMed] [Google Scholar]
- 63.Kobashigawa J, Mehra M, West L, et al. Report from a consensus conference on the sensitized patient awaiting heart transplantation. J Heart Lung Transplant. 2009;28:213. doi: 10.1016/j.healun.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hardinger KL. Rabbit antithymocyte globulin induction therapy in adult renal transplantation. Pharmacotherapy. 2006;26:1771. doi: 10.1592/phco.26.12.1771. [DOI] [PubMed] [Google Scholar]
- 65.Kho MM, Bouvy AP, Cadogan M, Kraaijeveld R, Baan CC, Weimar W. The effect of low and ultra-low dosages Thymoglobulin on peripheral T, B and NK cells in kidney transplant recipients. Transpl Immunol. 2012;26:186. doi: 10.1016/j.trim.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 66.Brokhof MM, Sollinger HW, Hager DR, et al. Antithymocyte globulin is associated with a lower incidence of de novo donor-specific antibodies in moderately sensitized renal transplant recipients. Transplantation. 2014;97:612. doi: 10.1097/TP.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Opelz G, Döhler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4:222. doi: 10.1046/j.1600-6143.2003.00325.x. [DOI] [PubMed] [Google Scholar]
- 68.Crespo-Leiro MG, Alonso-Pulpón L, Arizón JM, et al. Influence of induction therapy, immunosuppressive regimen and anti-viral prophylaxis on development of lymphomas after heart transplantation: data from the Spanish Post-Heart Transplant Tumour Registry. J Heart Lung Transplant. 2007;26:1105. doi: 10.1016/j.healun.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 69.O'Neill JO, Edwards LB, Taylor DO. Mycophenolate mofetil and risk of developing malignancy after orthotopic heart transplantation: analysis of the transplant registry of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25:1186. doi: 10.1016/j.healun.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 70.Marks WH, Ilsley JN, Dharnidharka VR. Posttransplant lymphoproliferative disorder in kidney and heart transplant recipients receiving Thymoglobulin: a systematic review. Transplant Proc. 2011;43:1395. doi: 10.1016/j.transproceed.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 71.Schubert S, Abdul-Khaliq H, Lehmkuhl HB, et al. Diagnosis and treatment of post-transplantation lymphoproliferative disorder in pediatric heart transplant patients. Pediatr Transplant. 2009;13:54. doi: 10.1111/j.1399-3046.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- 72.Schubert S, Renner C, Hammer M, et al. Relationship of immunosuppression to Epstein-Barr viral load and lymphoproliferative disease in pediatric heart transplant patients. J Heart Lung Transplant. 2008;27:100. doi: 10.1016/j.healun.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 73.Pollock-BarZiv SM, Allain-Rooney T, Manlhiot C, et al. Continuous infusion of thymoglobulin for induction therapy in pediatric heart transplant recipients: experience and outcomes with a novel strategy for administration. Pediatr Transplant. 2009;13:585. doi: 10.1111/j.1399-3046.2008.01035.x. [DOI] [PubMed] [Google Scholar]
- 74.Di Filippo S, Boissonnat P, Sassolas F, et al. Rabbit antithymocyte globulin as induction immunotherapy in pediatric heart transplantation. Transplantation. 2003;75:354. doi: 10.1097/01.TP.0000045223.66828.FA. [DOI] [PubMed] [Google Scholar]
- 75.Agha IA, Rueda J, Alvarez A, et al. Short course induction immunosuppression with thymoglobulin for renal transplant recipients. Transplantation. 2002;73:473. doi: 10.1097/00007890-200202150-00025. [DOI] [PubMed] [Google Scholar]
- 76.Büchler M, Longuet H, Lemoine R, et al. Pharmacokinetic and pharmacodynamic studies of two different rabbit antithymocyte globulin dosing regimens: results of a randomized trial. Transpl Immunol. 2013;28:120. doi: 10.1016/j.trim.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 77.Gaber AO, Matas AJ, Henry ML, et al. Antithymocyte globulin induction in living donor renal transplant recipients: final report of the TAILOR registry. Transplantation. 2012;94:331. doi: 10.1097/TP.0b013e31825a7d1f. [DOI] [PubMed] [Google Scholar]
- 78.Khanmoradi K, Knorr JP, Feyssa EL, et al. Evaluating safety and efficacy of rabbit antithymocyte globulin induction in elderly kidney transplant recipients. Exp Clin Transplant. 2013;11:222. doi: 10.6002/ect.2012.0211. [DOI] [PubMed] [Google Scholar]
- 79.Goland S, Czer LS, Coleman B, et al. Induction therapy with Thymoglobulin after heart transplantation: impact of therapy duration on lymphocyte depletion and recovery, rejection, and cytomegalovirus infection rates. J Heart Lung Transplant. 2008;27:1115. doi: 10.1016/j.healun.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 80.Aliabadi A, Grömmer M, Cochrane A, Salameh O, Zuckermann A. Induction therapy in heart transplantation: where are we now? Transpl Int. 2013;26:684. doi: 10.1111/tri.12107. [DOI] [PubMed] [Google Scholar]


