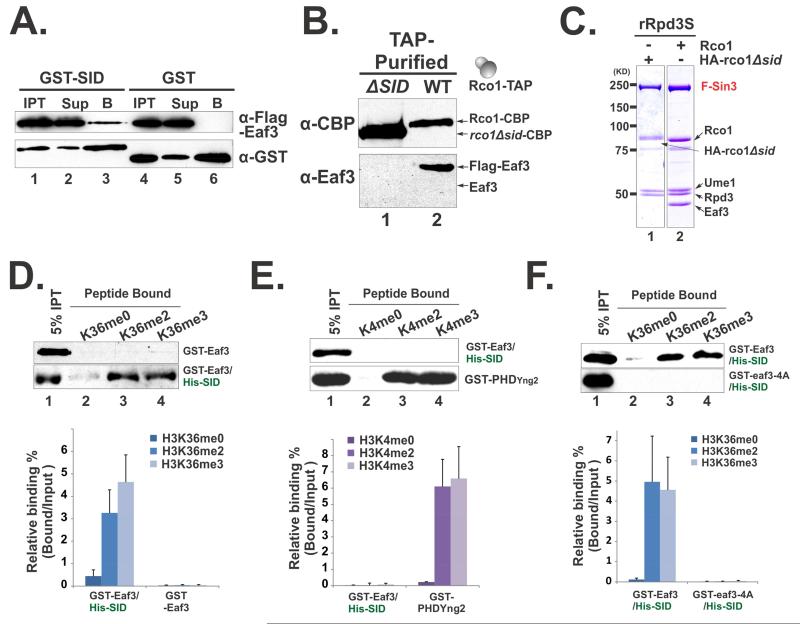
Figure 2. Eaf3 can be allosterically activated to recognize H3K36me.
(A-C) SID is required for incorporation of Eaf3 in Rpd3S; (A) GST-SID interacts with Flag-Eaf3 in vitro as shown by GST pull-down experiments. “IPT”-Input, “Sup”-Supernatant, “B”-Bound to beads; (B) Western blots of native Rpd3S that were TAP-purified from yeast strains YCR353(ΔSID) and YBL583(WT); (C) Coomassie staining of recombinant Rpd3S complexes purified from insect cells. (D-F) SID stimulates Eaf3 to recognize H3K36me preferentially. Histone peptide pull-down assays were performed using indicated proteins or protein complexes. The upper panels in each figures show representative western blot results, while the lower panels display quantification of the western results based on at least four independent experiments. Data are represented as mean ± SEM; (D) SID increases the binding of Eaf3 to H3K36 methylated peptides; (E) SID/Eaf3 heterodimer does not bind to H3K4 methylated peptides. The PHD domain of Yng2 was used as a positive control; (F) The elevated binding of SID/Eaf3 to H3K36 methylated peptides relies on the aromatic cage of Eaf3 CHD. pBL1290 was used to purify the cage mutant of SID/Eaf3, in which all four aromatic residues were mutated to alanine (GST-eaf3-4A). See also Figure S2.