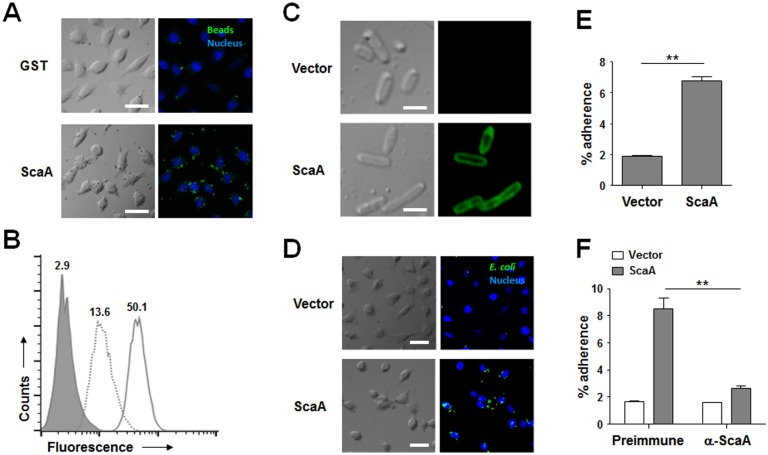
Fig 2. Adhesion function of ScaA.
(A) HeLa cells were incubated with fluorescent microbeads coated with GST or GST-ScaA (ScaA) for 1 h, washed extensively, and fixed. Cell-bound microbeads (green) were visualized by fluorescence microscopy after staining of cell nuclei (blue). Scale bars, 10 μm. (B) Relative binding of the microbeads coated with GST (dotted line) or GST-ScaA (thick line) to HeLa cells was quantified directly using fluorescence-activated cell sorter (FACS) analysis. The gray histogram represents unbound cells (cells not incubated with microbeads). (C) Immunofluorescence microscopy using an anti-ScaA antibody revealed the presence of ScaA on the surface of the recombinant E. coli (lower panels). Preimmune serum did not detect the recombinant protein (upper panels). Scale bars, 5 μm. (D) E. coli transformed with the pET28a vector or with pScaA was induced with IPTG and incubated with HeLa cells. After being washed to remove adherent bacteria, the cells were fixed, permeabilized, and stained with an anti-E. coli antibody (green) and ToPro-3 for nuclear staining (blue). Scale bars, 10 μm. (E) CFU-based quantification of adherent E. coli transformed with the vector or pScaA was performed. The results are presented as percentages of adherent bacteria relative to the total bacterial input. Data are representative of three independent assays for each of the host cells. **, p < 0.01. (F) Inclusion of anti-ScaA serum in the medium (α-ScaA) significantly inhibited adhesion of E. coli expressing ScaA into host cells. After addition of anti-ScaA or preimmune serum into infection media, CFU-based quantification of adherent E. coli transformed with the vector or pScaA was performed. **, p < 0.01.