Figure 3. SKP2 suppresses K63-linked ubiquitination of JARID1B to elevate H3K4me3 in human prostate cancer cells.
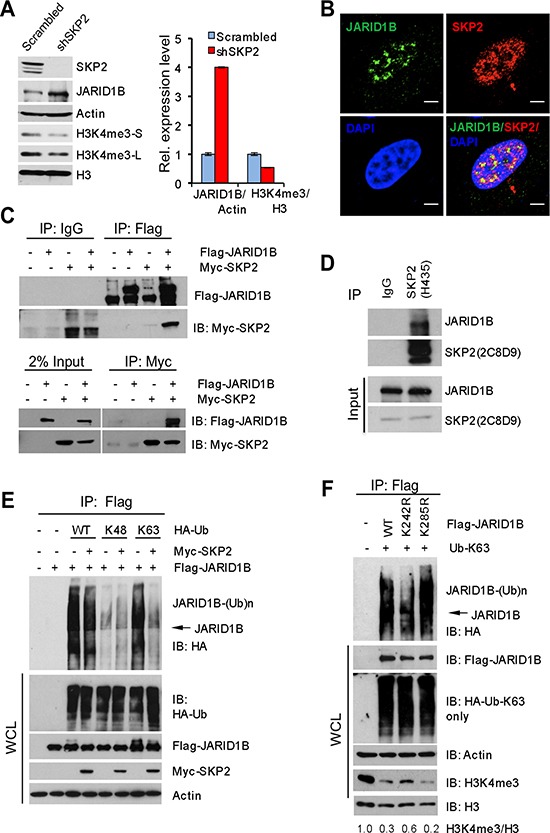
(A) Western blot analysis shows JARID1B elevation and H3K4me3 reduction in PC3 cells upon SKP2 knockdown by shRNA. S - short exposure, L - long exposure. Right panel: quantification analysis on the abundance of H3K4me3 and JARID1B proteins from the left panel. Error bars represent means ± SD. (B) Immunofluorescence images show the co-localization of endogenous SKP2 and JARID1B proteins in PC3 cells. Scale bars represent 10 μm. (C) Co-immunoprecipitation analysis shows a physical interaction between JARID1B and SKP2 proteins in HEK293T cells. (D) Co-immunoprecipitation analysis shows a physical interaction between endogenous JARID1B and SKP2 proteins in PC3 cells. (E) In vivo ubiquitination assay shows that a reduction of K63-linked ubiquitination of JARID1B upon addition of SKP2 in HEK293T cells. Cells were transfected with Flag-JARID1B, Myc-SKP2, along with various HA-ubiquitin (HA-Ub) constructs. K48 and K63 indicate HA-Ub-K48-only and HA-Ub-K63-only, respectively. WCL indicates whole cell lysates. (F) JARID1B mutation affects H3K4me3 levels by altering the ubiquitination. In vivo ubiquitination assay was performed in HEK293T cells transfected with HA-Ub-K63-only, along with various JARID1B constructs (Flag-JARID1B, Flag-JARID1B-K242R and Flag-JARID1B-K285R) (see Supplementary Figure S4). Bottom panel: Western blot analysis shows the effects of JARID1B WT and JARID1B mutants on the changes of endogenous protein levels of H3K4me3 through the K63-linked ubiquitination in cells. WCL indicates the whole cell lysates.
