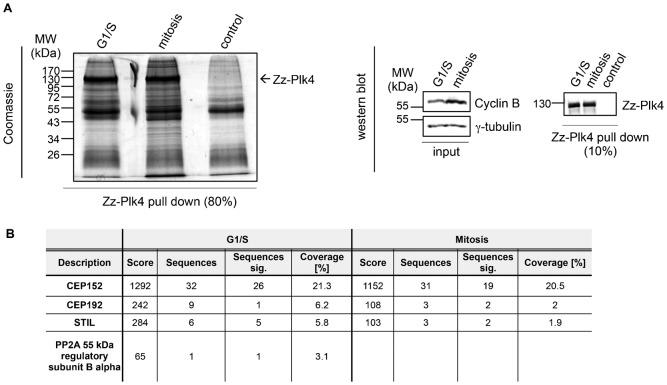
Fig. 1. Identification of STIL as a Plk4 interacting protein.
(A) Bacterially purified Zz-Plk4 (N-terminal Zz-tag and C-terminal His-tag) was incubated with G1/S or mitotically arrested (double thymidine block and release) HeLa Kyoto cell lysates. Cell lysates alone served as control. Plk4 was immunoprecipitated via its Zz-tag and eluted with its interaction partners. Coimmunoprecipitating proteins were detected by staining with Colloidal Coomassie (left panel) and analyzed by mass spectrometry. Western blotting (right panel) using anti-His antibodies was performed to detect Zz-Plk4 by its His-tag in elution fractions. Cyclin B abundance was used to determine the cell cycle stages of the lysates and anti γ tubulin detection served as a loading control. (B) Mass spectrometry analysis of Zz-Plk4 pull down identified known Plk4 interaction partners, substrates or regulators and STIL as a novel Plk4 interaction partner.