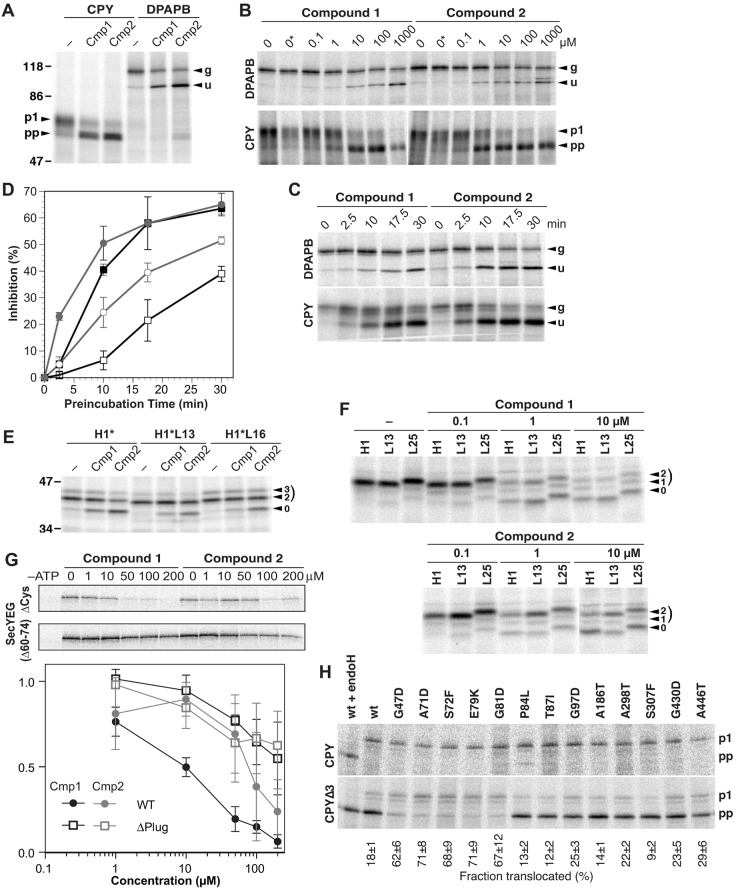
Fig. 5.
The compounds inhibit translocation by the yeast, human, and bacterial Sec61/SecYEG translocons. (A) Yeast cells expressing CPY or DPAPB (typical post- and co-translationally translocated substrates, respectively) were preincubated for 30 min with 1% DMSO with or without decadepsipeptide compound 1 (Cmp1) or cotransin heptadepsipeptide compound 2 (Cmp2) to a final concentration of 100 µM, labeled for 5 min with [35S]methionine in the continued presence or absence of the compounds, and analyzed by immunoprecipitation, gel electrophoresis and autoradiography. p1 and pp indicate glycosylated proCPY in the ER lumen and untranslocated preproCPY. g and u indicate the glycosylated and unglycosylated forms of DPAPB. The position of molecular mass standards with their weight in kDa is indicated. (B) Dose-dependence of translocation inhibition for DPAPB and CPY was analyzed by metabolic labeling as above, using the indicated concentrations of compound 1 or 2 after preincubation for 30 min. 0 and 0* indicate labeling without or with DMSO, respectively, in the absence of inhibitors. (C) The time-course of inhibition was analyzed by metabolic labeling of DPAPB and CPY, as above, using a fixed concentration of 10 µM compound 1 or compound 2, and the indicated preincubation times of 0–30 min. (D) Quantification of translocation inhibition experiments as in C. Squares are used for DPAPB, circles for CPY, open symbols for compound 1, and filled symbols for compound 2. The mean±s.d. of two independent experiments are shown. (E) H1*, a protein derived from the mammalian type II membrane protein H1, with its natural signal-anchor sequence, or generic hydrophobic sequences composed of Leu13 or Leu16 were expressed in yeast and analyzed for inhibition of translocation by compounds 1 and 2 as in A. The unglycosylated, and the two- and three-fold glycosylated forms are indicated by 0, 2 and 3, respectively. (F) To analyze the effect on translocation in mammalian cells, COS-1 cells were transfected to express the asialoglycoprotein receptor H1 or derivatives in which the hydrophobic core of its signal-anchor was replaced by generic sequences of Leu13 or Leu25. Cells were labeled for 30 min with [35S]methionine in the presence or absence of the indicated concentrations of compound 1 or 2. H1 and its derivatives were immunoprecipitated and analyzed by gel electrophoresis and autoradiography. (G) To analyze inhibition of translocation in E. coli, purified SecA and SecYEG derivatives [full-length lacking cysteine residues (ΔCys and WT) or with deletion of plug residues 60–74 (Δ60-74 and ΔPlug)] were reconstituted into proteolipsomes. Radiolabeled proOmpA-DHFR (a post-translationally translocated fusion protein) was incubated in the presence of the indicated compound concentrations with ATP or without (–ATP). Translocation of the substrate was measured by proteinase K treatment followed by gel electrophoresis and autoradiography. The presented dose–response curves are mean±s.d. based on quantification of three experiments. (H) Sec61p mutants isolated by their resistance to 30 µM compound 1 were analyzed for their ability to suppress the translocation defect of CPYΔ3 (CPY with a mutant signal sequence lacking three apolar residues) by analyzing metabolic labeling of cells expressing CPYΔ3 or, as a control, CPY for 5 min, followed by immunoprecipitation, SDS-gel electrophoresis, and autoradiography. Glycosylation to the p1 forms indicates translocation into the ER lumen, whereas the unglycosylated preproCPY form is cytosolic. As a control, the material in the first lane was deglycosylated by endoglycosidase H (endoH) digestion. The fraction of translocated products is indicated as the percentage of the total (mean±s.d.; n = 3).