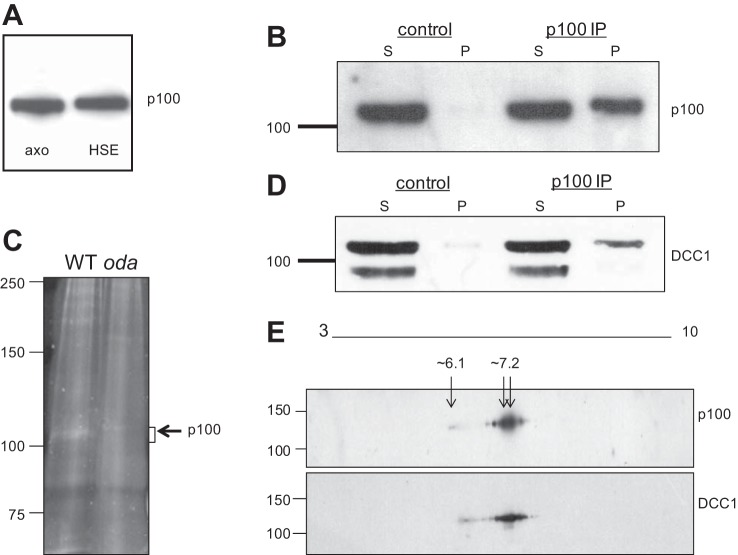
Fig. 3.
The p100 phosphoprotein is DCC1. A: isolated WT axonemes containing p100 (axo) were extracted with high-salt buffers, and the fractions were analyzed by Western blot using the anti-phosphothreonine antibody. p100 is solubilized from the axoneme with high salt and is detected in the high-salt extract (HSE). B: p100 is immunoprecipitated from HSEs with the anti-phosphothreonine antibody but not with control IgG. C: protein-stained gel showing a band at the expected size for p100 in WT immune complexes, which is absent in immune complexes from an ODA mutant, oda3. The p100 region in both WT and oda3 were excised and analyzed by tandem MS/MS. D: blots in B were stripped and reprobed with the DCC1 antibody, which shows a band for DCC1 in the immune pellet (p100 immunoprecipitation, IP) which comigrates with the p100 band. E: p100 and DCC1 comigrate on 2D gels. WT axonemes were separated on 2D gels followed by Western blotting with the anti-phosphothreonine and DCC1 antibodies. The spot patterns detected by both antibodies comigrate at the same molecular weight and pI range (∼6.1–7.2).