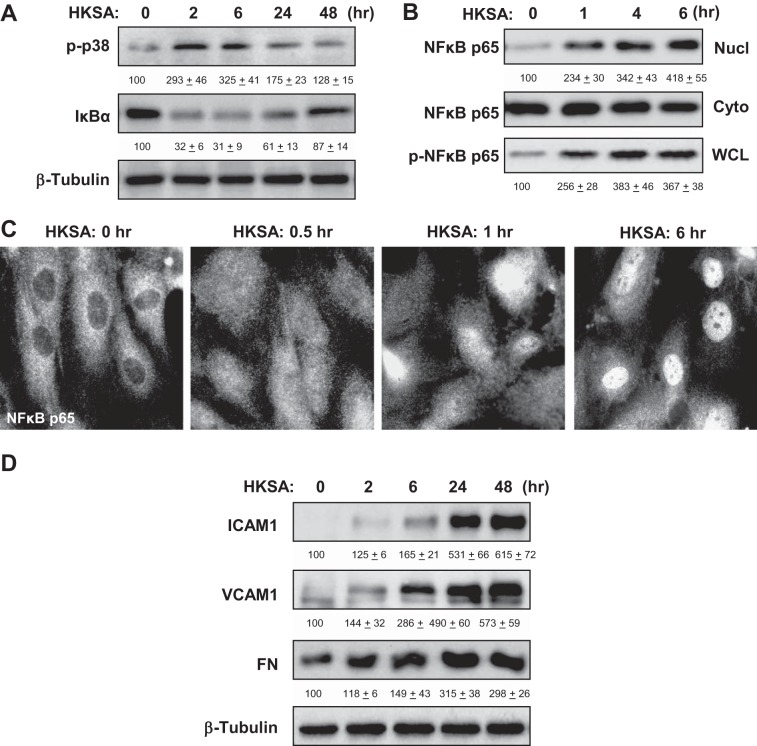
Fig. 2.
Effects of HKSA on inflammatory signaling. A: human pulmonary artery endothelial cells (HPAEC) were challenged with HKSA (5 × 108 bacterial particles/ml) for indicated periods of time. Phosphorylation of p38 MAP kinase and degradation of IκBα was detected by immunoblotting with corresponding antibodies. Equal protein loading was confirmed by determination of β-tubulin content in total cell lysates. B: after EC treatment with HKSA, the content of NF-κB p65-subunit in nuclear (Nucl) and cytosolic (Cyto) fractions was performed using immunoblotting. The level of phosphorylated NF-κB p65 in the whole cell lysates (WCL) was monitored by Western blot with phospho-specific NF-κB p65 antibody. C: HKSA-induced nuclear translocation of NF-κB was visualized by immunofluorescence staining of EC culture with NF-κB antibody. D: time-dependent expression of ICAM1, VCAM1, and fibronectin (FN) caused by HKSA stimulation of pulmonary was monitored by immunoblotting. Equal protein loading was confirmed by determination of β-tubulin content in total cell lysates. The results of Western blot quantitative densitometry are presented as means ± SD of 5 independent experiments.