Figure 1.
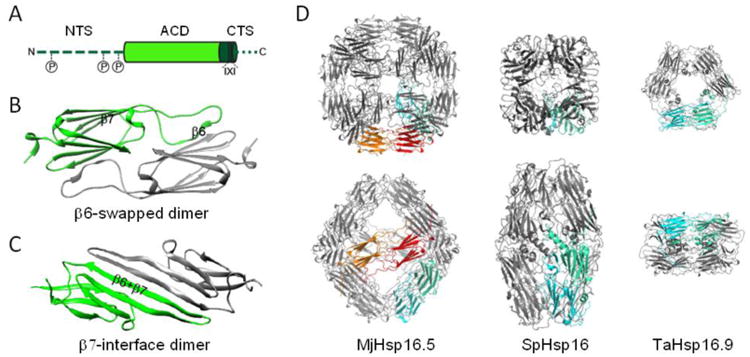
(A) Domain organization of sHsps. N-terminal sequence (NTS; dark green, dashed line), α-crystallin domain (ACD; light green), C-terminal sequence (CTS; dark green with the conserved I-X-I motive in cylinder form, and remainder as a dotted line). As indicated, up to three phosphorylation sites exist in the NTS of some sHsps as discussed in the text. (B) Structure of a β6-swapped dimer of the ACD of M. jannaschii Hsp16.5 (X-ray crystallography, PDB: 1SHS; [45]). The ACDs of individual protomers are colored green and gray. (C) Structure of a β7-interface dimer of the ACD of human αB-crystallin (solid state NMR, PDB: 2KLR; [49]). (D) To scale comparison of the three available oligomeric crystal structures of sHsps. One dimeric building block is marked in green-cyan to highlight the variable interconnections of the dimers in the respective structures. MjHsp16.5; M. jannashii Hsp16.5 representing a 24mer [45]. The orange-red highlighted dimeric building block additionally highlights the equatorial protein axis forming an octahedron. SpHsp16; Schizosaccharomyces pombe Hsp16 representing a 16mer ellipsoid composed of two half-spheres of four dimers [46]. TaHsp16.9; Tritium aestivum (wheat) Hsp16.9 representing a 12mer of two stacked rings [40].
