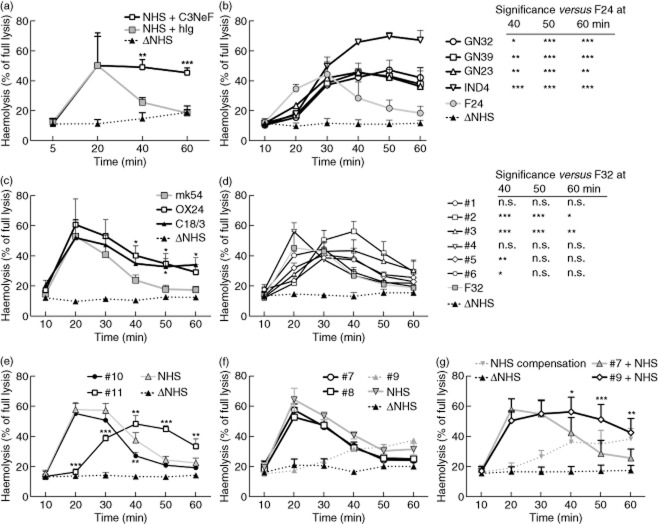
Fig 4.
Screening for factors prolonging stability of the alternative convertase. (a) Time of maximal convertase activity (Tmax) profiles of the alternative complement convertase obtained for heat-inactivated normal human serum (Δ NHS, negative control) or normal human serum (NHS) supplemented with 30 μg/ml of C3 nephritic factor (C3NeF) (NHS+C3NeF) or human immunoglobulin (Ig) [NHS+human immunoglobulin (hIg)]. (b) Tmax profiles of sera positive for C3NeF (black connecting lines) and control, individual serum from healthy volunteer (grey connecting line), both mixed 1:1 with NHS at 2·5%. (c) Experiments similar to those in (a) but NHS was supplemented with 25 μg/ml of mouse monoclonal antibodies (mAbs) OX24 or C18/3 (both are FH function-blocking mAbs) or mouse control antibody mk54. (d) Tmax profiles of sera positive for autoantibodies against complement factor H (FHaAbs) (black connecting lines) and control, individual serum from healthy volunteer (grey connecting line). (e.f) Tmax profiles of convertases formed by sera containing mutated complement components, when tested unmixed at 5% concentration. Data shown are the average of at least three independent experiments, error bars represent standard deviation and significance is indicated by *P < 0·05, **P < 0·01 and ***P < 0·001, according to two-way analysis of variance (anova), respectively. Originally, three more individual sera from healthy volunteers were included to the data presented in (b) and (d) but they did not differ significantly from each other at any time-points, so were removed for better visibility of the graphs.