Abstract
Background
Detection of allergen-induced basophil activation by flow cytometry has been shown to be a useful tool for allergy diagnosis.
Objective
The aim of this study was to assess the potential of this technique for the diagnosis of pediatric cow milk allergy.
Material and methods
The quantification of total and specific IgE and basophil activation test were performed to evaluate cow milk allergic (n = 9), and non-allergic children (n = 15).
Results
Allergen-induced basophil activation was detected as a CD203c up-regulation. The expression of CD203c antigen on basophils was measured with flow cytometry. The antigen CD203c was detected on 15.4 ± 10.2% basophils from allergic children after incubation with specific allergens in concentration 1:10, whereas in the control group there were 3.0 ± 1.5% of basophils positive for this molecule (P < 0.05). Stimulation with allergen diluted 1:500 resulted in activation of 15.3 ± 11.2% of basophils in allergic children and 3.8 ± 2.3% of cells in the control group (P < 0.05). Positive results of an allergenicity test (above the cut- off level of 10%) were obtained in 7 out of the 9 allergic children. In 5 cases, the cutoff level was reached with both dilution of allergens (1:10 and 1:500). In 1 patient, positive stimulation was observed after stimulation with allergen diluted 1:10 and in another case only 1:500 resulted in stimulation of more then 10% of basophils. In no child of the control group, stimulation above 10% was noted.
Conclusions
This study demonstrates that the analysis of allergen-induced CD203c up-regulation with flow cytometry is a reliable tool for the diagnosis of cow milk allergy in pediatric patients, with sensitivity similar to routine diagnostic tests and a higher specificity.
Keywords: cow milk allergy, basophil activation test, CD203c, diagnosis, children
Introduction
Allergy to cow milk is very common in young children and infants. Digested fractions of the milk proteins may trigger pathological immune response and induce the production of antibodies [1,2]. The evaluation of a child suspected of food allergy includes a combination of the several diagnostic tools [1-3]. The diagnosis is made on the basis on medical history and physical examination, prick skin testing, patch testing, in vitro tests, elimination diet, and food challenges. None of the laboratory tests have reached adequate diagnostic accuracy, and the food challenge is connected with a substantial risk of exacerbation of allergy symptoms. Skin prick and patch tests are the most common in vivo tests as the first line investigation. Their positive predictive accuracies, the correlation between the positivity of the test, and the presence of cow milk allergy, vary between 69 and 100% and the negative predictive values between 20 to 86% [3]. Prick tests may be negative and the specific IgE absent especially in children with delayed reactions, a clinical problem which may be overcome by using the atopy patch test (APT) [4]. APT involves prolonged contact of the allergenic food extract with the intact skin under occlusion for 48 h. Positive reactions to patch tests consist of erythema and induration. The measurement of the concentration of food-specific IgE antibodies may be useful when identifying a subset of patients highly likely (> 95% risk) to experience clinical reactions to milk [5].
A high level of milk specific IgE is useful in diagnosing symptomatic allergy to milk in the pediatric population, but the results remain inconclusive [1,6,7]. In most cases, a placebo-controlled food challenge, a definitive diagnostic test for cow milk allergy, is still required for a subset of patients [1,2]. Newly developed, functional in vitro tests for allergic reactions are focused on circulating basophils and may overcome the need for food challenge tests in some pediatric patients. The first approach to basophil functional studies has been the histamine release test. However, its clinical benefit remains controversial due to insufficient sensitivity and specificity [8,9]. The next generation of basophil functional test is based on flow cytometry, as a tool to monitor basophil activation upon allergen challenge by detecting surface expression of activation markers [9]. Basophils and mast cells are the main effector cells responsible for atopic reactions. A large panel of mediators including cytokines, histamine, and tryptase, is released from the cell upon degranulation [8,9]. In addition, changes in the expression of basophil surface molecules are observed: some antigens appear de novo (CD63, CD107a) and others significantly increase their expression (CD13, CD203c). The CD203c antigen is one of the most important markers of basophil activation. This antigen belongs to the type II transmembrane protein family and is a multifunctional ecto-enzyme called ectonucleotide pyrophosphatase phophodiesterase 3 (E-NPP3) [9,10] that catalyzes the cleavage of a number of molecules including deoxynucleotides and nucleotide sugars. In addition, ENPP3 contains a somatomedin B-like domain and a cell adhesive motif, but their potential functions remain unknown.
Among leukocytes, CD203c appears to be selectively expressed in the basophil/mastocytes lineage [9]. Its expression increases after basophil degranulation and, therefore, is a reliable marker of basophil activation in the IgE-dependent process. Resting basophils are characterized by a low expression of this protein which rapidly increases upon activation [9]. Another molecule that can facilitate basophil recognition is CRTH2 (chemoattractant receptor-homologous molecule expressed on Th2 cells) as it is highly expressed on basophils. CRTH2 is present only on cells associated with Th2 responses (Th2 lymphocytes, eosinophils and basophils). Consequently, a new three-color flow cytometric protocol (PE-CD203c/FITCCRTH2/PC5-CD3) for the assessment of allergen-induced basophil activation has been developed [11,12]. CRTH2 and CD203c staining allows for an easy distinguishing of basophils from other cells in samples of whole blood.
The goal of this study was to assess the usefulness of a basophil activation test, based on the CD203c expression, in the diagnosis of cow milk allergy in children.
Materials and methods
The experiments were approved by the Ethics Committee of Warsaw Medical University in Warsaw, Poland. The blood for the analysis in this study was collected with parental approval. Nine infants and children aged 5-35 months (median 11 months), suffering from cow milk allergy, confirmed with in vitro specific IgE test positive to milk allergens (Unicap, Pharmacia, Uppsala, Sweden), served as the study group. The blood had been drawn before milk-free diet was introduced. All allergic children showed features of eczema or gastrointestinal symptoms after ingestion of cow milk. 15 healthy, non-allergic controls aged 5-95 months (median 26 months) were included in the control group. None of the subjects were treated with antihistamine drugs or oral corticosteroids.
Basophil Activation Tests
All tests were carried out within two hours from blood collection. A residual blood sample of 400 μl, remaining from routine blood count analysis was used for the basophil activation test. CD203c induced expression was evaluated using the Allergenicity Kit (Beckman Coulter, U.K.) according to the manufacturer's instructions. Milk proteins suspension for prick tests (Allergopharma, Reinbek, Germany) was diluted 1:10 and 1:500 in phosphate buffered saline (PBS). EDTA-anticoagulated peripheral blood aliquots (100 μl) stained with 20 μl of mixture of monoclonal antibodies (CRTH2-FITC, CD203c-PE, CD3-PC7) and Activation Solution (100 μl) were stimulated (37°C) for 15 min with 20 μl of optimal dilution of allergens; antibody directed against the high affinity IgE receptor (FcεRI) (Beckman Coulter, U.K.) was used as a positive control and PBS as a negative control. After incubation, the reaction was stopped with Stop Solution. Erythrocytes were lysed with Lysing Solution for 10 min at room temperature (RT). Suspension was centrifuged (5 min, 300 x g) after lysing, washed with PBS, once more centrifuged and resuspended in 500 μl Fixative Solution. Leukocytes were analyzed using a five-color flow cytometer (Cytomics FC500, Beckman Coulter, U.K.). During acquisition, basophils were selected as CD203c positive/CRTH2 high/CD3 negative population using FL1/FL2 and SS/FL5 dot plots. In the negative control, the threshold for positivity was set at less than 5% of activated cells according to the literature data [1,9]. In the positive control, sensitivity for the IgE dependent reaction was verified. The threshold for a positive reaction was settled at less than 15% of activated cells, according to literature data and the investigators' previous studies [11,12].
Statistical Analysis
Results are presented as means ± SD. A comparison of the results from different groups was performed using Mann-Whitney U test. P < 0.05 was considered significant. Specificity and sensitivity were calculated according to following equations:
sensitivity = TP/(TP+FN) × 100% and specificity = TN/(TN+FP) × 100%; where: TP - true positive, FD false positive, TN - true negative, FN - false negative results.
Results
The CD203c expression on basophils was evaluated to assess patients' sensitivity to cow milk antigen stimulation. The mean fluorescence of fluorochrome bound to CD203c was measured before and after allergenic stimulation. As a negative control, fluorescence of spontaneously activated basophils was measured. As a positive control, fluorescence of IgE stimulated basophil was estimated (Figure 1). The cut-off level was set at > 10% CD203c positive cells.
Figure 1.
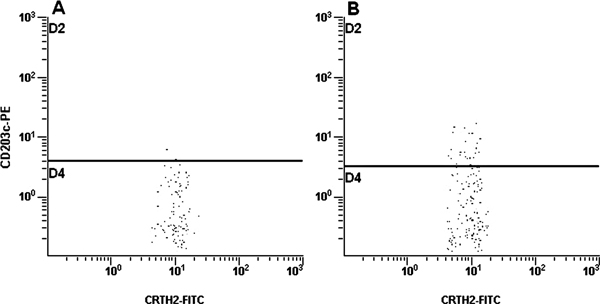
Expression of CD203c in negative (A) and positive (B) controls.
The mean percentages of activated basophils in the allergic children were 3.8 ± 0.7% (negative control) and 22.2 ± 13.9% (positive control). Accordingly, in the group of control children, the mean percentage of cells with CD203c expression was 3.6 ± 1.2% (negative control) and 31.7 ± 16.5% (positive control). The mean fluorescence of non-stimulated cells in the allergic children was 13.3 ± 7.3 MFC and that in the control children was 17.4 ± 9.7 MFC; the difference being insignificant. The presence of CD203c was detected on 15.4 ± 10.2% basophils from allergic children after incubation with specific allergens in the concentration 1:10, whereas in the control group 3.0 ± 1.5% of basophils were positive for this molecule (P < 0.05). Stimulation with the allergen diluted 1:500 resulted in the activation of 15.3 ± 11.2% of basophils in the allergic children and 3.8 ± 2.3% of cells in the control group (P < 0.05).
Positive results of the allergenicity test were obtained in 7 out of the 9 allergic children. In 5 cases, the cut-off level was reached with both dilutions of allergens (1:10 and 1:500). In 1 patient, positive stimulation was observed after stimulation with the allergen diluted 1:10 and in another case only 1:500 resulted in stimulation of more than 10% of basophils. In 2 patients, significant basophil activation was not observed. In no child of the control group was the stimulation above 10% noted. In 4 healthy children, the percentage of activated basophils was 5-10%. The individual results are summarized in Tables 1 and 2.
Table 1.
Percentage of activated basophils in the group of cow milk allergic children.
| No | Activated cells in cells in negative control (%) | Activated cells in positive control (%) | Activated cells after stimulation with allergens diluted 1:10 (%) | Activated cells after stimulation with allergens diluted 1:500 (%) | Fluorescence of spontaneously activated basophils (MFC units) |
|---|---|---|---|---|---|
| 1 | 2.8 | 51.4 | 1.7 | 5.3 | 4.9 |
| 2 | 3.5 | 29.9 | 33.1 | 37.3 | 6.3 |
| 3 | 4.1 | 15.4 | 16.9 | 3.7 | 13.3 |
| 4 | 3.1 | 5.0 | 9.3 | 4.6 | 11.0 |
| 5 | 4.7 | 16.2 | 15.6 | 16.7 | 18.7 |
| 6 | 4.5 | 17.5 | 23.1 | 26.2 | 25.2 |
| 7 | 4.1 | 33.3 | 5.9 | 11.8 | 11.7 |
| 8 | 3.9 | 18.2 | 13.1 | 19.9 | 6.4 |
| 9 | 3.5 | 13.1 | 21.2 | 11.8 | 22.1 |
The results above the cut-off level are bolded.
Table 2.
Percentages of activated basophils in the control group.
| No | Activated cells in cells in negative control (%) | Activated cells in positive control (%) | Activated cells after stimulation with allergens diluted 1:10 (%) | Activated cells after stimulation with allergens diluted 1:500 (%) | Fluorescence of spontaneously activated basophils (MFC units) |
|---|---|---|---|---|---|
| 1 | 4.7 | 67.3 | 1.9 | 3.1 | 16.0 |
| 2 | 1.9 | 44.2 | 3.3 | 7.3 | 7.6 |
| 3 | 4.3 | 20.2 | 2.8 | 0.8 | 22.0 |
| 4 | 4.7 | 23.8 | 2.7 | 7.5 | 20.0 |
| 5 | 2.6 | 20.7 | 0.6 | 1.0 | 7.0 |
| 6 | 3.9 | 16.1 | 3.6 | 5.0 | 18.9 |
| 7 | 1.2 | 15.4 | 1.9 | 1.6 | 11.8 |
| 8 | 2.4 | 11.8 | 0.0 | 0.0 | 18.5 |
| 9 | 3.3 | 16.2 | 5.6 | 5.2 | 34.5 |
| 10 | 4.7 | 42.3 | 4.1 | 3.8 | 32.5 |
| 11 | 3.3 | 29.7 | 2.3 | 3.1 | 6.8 |
| 12 | 5.4 | 29.0 | 4.4 | 5.7 | 32.7 |
| 13 | 3.8 | 52.7 | 4.1 | 4.1 | 14.5 |
| 14 | 4.6 | 37.0 | 4.6 | 3.8 | 5.9 |
| 15 | 3.5 | 49.3 | 3.9 | 4.6 | 12.1 |
The percentage of Th2 cells among lymphocytes in both allergic and non-allergic children was calculated. In the children allergic to cow milk, the percentage of Th2 lymphocytes was 0.12-2.8% (median 0.33) and that in the control group was 0.02-2.8% (median 0.48); the difference being insignificant. These results are presented in Table 3. Diagnostic accuracy of the basophil activation test is presented in Table 4. The test sensitivity was 66.7% and the specificity was 100%.
Table 3.
Percentage of Th2 cells among lymphocytes (%).
| No | Allergic children children | Healthy children children |
|---|---|---|
| 1 | 0.2 | 0.4 |
| 2 | 0.3 | 0.3 |
| 3 | 0.4 | 0.4 |
| 4 | 0.4 | 0.6 |
| 5 | 0.3 | 0.1 |
| 6 | 0.3 | 0.4 |
| 7 | 0.1 | 0.0 |
| 8 | 2.3 | 0.3 |
| 9 | 2.8 | 1.2 |
| 10 | -- | 0.7 |
| 11 | -- | 0.5 |
| 12 | -- | 0.6 |
| 13 | -- | 2.8 |
| 14 | -- | 2.6 |
| 15 | -- | 0.8 |
Table 4.
True positive (TP), false positive (FP), true negative (TN), and false negative (FN) results.
| Basophil activation test | Presence of cow milk allergy | |
|---|---|---|
| Yes | No | |
| Positive | TP (n = 12) | FP(n = 0) |
| Negative | FN (n = 6) | TN (n = 30) |
| Together | TP + FN (n = 18) | FP + TN (n = 30) |
Discussion
Basophil activation with specific allergens has proven to be useful for diagnosis of pollen, latex, and food allergy [13]. CD203c up-regulation is more or less specific to the cross linking of FcεRI. Hence, as CD203c is rapidly up-regulated after allergen challenge, it has been proposed as a new tool for allergy diagnosis [9,13-15]. Our study demonstrates that the analysis of allergen-induced CD203c up-regulation by flow cytometry is a reliable tool for diagnosis of cow milk allergy in pediatric patients, with sensitivity similar to routine diagnostic tests and a higher specificity than that of specific IgE determination. Therefore, the basophil activation test is a highly efficient in confirming cow milk allergy in children. Several authors have studied the diagnostic value of basophil activation test in food allergy. Ocmant et al [16] have found sensitivity of 89.5% and specificity of 97.1% in peanut allergy and that of 62.5% and 96.4%, respectively, in egg allergy. Tokuda et al [17] have confirmed 85.0% sensitivity and 77.0% specificity in wheat allergy. In the present study, two of the examined patients with confirmed cow milk allergy presented a negative result of the basophil activation test. At least in one case, this result may be explained by a relatively low basophil count in the peripheral blood. In this patient, the response to positive control also was low (4.95%). The cause of the unresponsiveness in another case is not known. As 40-50% of milk allergy is non-IgEmediated [18], we may speculate that in this patient the allergy was induced by IgE-unrelated mechanisms. Two of the examined children responded to activation with only one solution of the allergen. It confirms the opinion of others that different concentrations of allergen may stimulate or block basophil response [19-21]. Interestingly, two of the control children also responded in a concentration-dependent manner; however, in neither case the obtained result exceeded the cut-off value. We also observed in a child from the control group that anti-IgE stimulation (positive control) did not result in increased expression of CD203c on basophils. This may be explained by disturbances in intracellular signaling commonly observed in around 5% of the general population (non-responders) [11]. On the other hand, the identification of basophils using prior protocols relied on a single IgE-labeling, showed that FcεRI expression can vary considerably on cell surfaces from one patient to another [22]. That is why in some cases basophils were difficult or impossible to be stimulated by anti-IgE monoclonal antibodies.
In the present study we also showed that there was no difference between the Th2 populations of cells in the allergic and non-allergic children. The lack of difference may be explained by the immaturity of the immune system in young children. Moreover, CRTH2-staining protocol, illustrated its superiority with respect to basophil recovery. Easy recognition of basophils and a reliable assessment of their activation make this protocol the most reliable tool for investigating basophil activation by flow cytometry. Since CRTH2 is also a marker of Th2 cells and eosinophils, it may become a promising tool for flow cytometry, providing a direct overview of cells involved in 'Th2 diseases', such as allergy [12,16,17].
It is a common observation that food allergy is a disease of young children, which in most cases is outgrown. The majority of children develop tolerance within the first 3-5 years of life [23]. The two foods responsible for most of these reactions are cow milk and hen eggs. It is then obvious that a reliable test to predict loss of sensitivity is needed. Children who acquire tolerance can continue to have positive skin test results for years, and therefore skin tests are not predictive of outgrowing the food sensitivity [23,24]. A positive double-blind, placebo-controlled food challenge is the gold standard for the diagnosis of food allergy [1,2]. After initiating a cow milk restriction diet, the clinical manifestations triggered by the first challenge are difficult to predict. In contrast, if the first challenge fails, further ones may result in severe symptoms. One must also be prepared to conduct challenges in a safe environment with available facilities for resuscitation. Rubio et al [25] have shown the benefit of a basophil activation test in predicting the child's reaction to the oral challenge, evaluating and comparing the specific IgE and skin prick test results. The authors used allergen-induced basophil activation based on CD63-upregulation by flow cytometry. The test had efficiency of 90%, sensitivity of 91%, and specificity of 90%, and positive and negative predictive values were 81% and 96%, respectively, in detecting persistently allergic patients [25]. According to this observation, a basophil activation test may be a useful and safe tool in predicting outgrowing the food sensitivity in children. To date, there are no good indices to predict when and in whom this occurs.
We conclude that a basophil activation test presents with high specificity and relatively low sensitivity in cow milk allergy in children. Basophil activation depends on the concentration of the allergen. As flow cytometry is a valuable tool for the analysis of many different cell types and can be used to identify specific populations of cells, even when present in low numbers, it seemed to be suitable for a study of allergen-induced basophil degranulation. Th2 analysis is not a good parameter discriminating allergic and non-allergic children.
Conflicts of interest
The authors declare that they have no competing interests.
References
- de Boissieu D, Dupont C. Time course of allergy to extensively hydrolyzed cow milk proteins in infants. J Pediatr. 2000;136:119–20. doi: 10.1016/S0022-3476(00)90063-5. [DOI] [PubMed] [Google Scholar]
- de Boissieu D, Dupont C. Allergy to extensively hydrolyzed cow milk proteins in infants: safety and duration of amino acid-based formula. J Pediatr. 2002;41:271–3. doi: 10.1067/mpd.2002.126299. [DOI] [PubMed] [Google Scholar]
- Fiocchi A, Bouygue GR, Restani P, Bonvini G, Startari R, Terracciano L. Accuracy of skin prick tests in IgE-mediated adverse reactions to bovine proteins. Ann Allergy Asthma Immunol. 2002;89(6 Suppl 1):26–32. doi: 10.1016/s1081-1206(10)62119-9. [DOI] [PubMed] [Google Scholar]
- de Boissieu D, Waguet JC, Dupont C. The atopy patch tests for detection of cow milk allergy with digestive symptoms. J Pediatr. 2003;142:203–5. doi: 10.1067/mpd.2003.92. [DOI] [PubMed] [Google Scholar]
- Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol. 1997;100:444–51. doi: 10.1016/S0091-6749(97)70133-7. [DOI] [PubMed] [Google Scholar]
- Isolauri E, Turjanmaa K. Combined skin prick and patch testing enhances identification of food allergy in infants with atopic dermatitis. J Allergy Clin Immunol. 1996;97:9–15. doi: 10.1016/S0091-6749(96)70277-4. [DOI] [PubMed] [Google Scholar]
- Heine RG, Elsayed S, Hosking CS, Hill DJ. Cow milk allergy in infancy. Curr Opin Allergy Clin Immunol. 2002;2:217–25. doi: 10.1097/00130832-200206000-00011. [DOI] [PubMed] [Google Scholar]
- Hamilton RG, Adkinson NF. In vitro assays for the diagnosis of IgE-mediated disorders. J Allergy Clin Immunol. 2004;114:213–25. doi: 10.1016/j.jaci.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Boumiza R, Debard AL, Monneret G. The basophil activation test by flow cytometry: recent developments in clinical studies, standardization and emerging perspectives. Clin Mol Allergy. 2005;3:9–13. doi: 10.1186/1476-7961-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhring HJ, Seiffert M, Giesert C, Marxer A, Kanz L, Valent P, Sano K. The basophil activation marker defined by antibody 97A6 is identical to the ectonucleotide pyrophosphatase/phosphodiesterase 3. Blood. 2001;97:3303–5. doi: 10.1182/blood.V97.10.3303. [DOI] [PubMed] [Google Scholar]
- Potapinska O, Gorska E, Zawadzka-Krajewska A, Kulus M, Wasik M, Demkow U. The usefulness of CD203c expression measurement on basophils after activation with grass pollen and Dermatophagoides pteronyssinus antigens. Preliminary study. Pneumonol Alergol Pol. 2009;77:138–44. [PubMed] [Google Scholar]
- Potapinska O, Demkow U, Wasik M. Flow cytometric basophils activation test as a method of allergy diagnosis. Pneumonol Alergol Pol. 2009;77:152–8. [PubMed] [Google Scholar]
- Gonzalez-Muñoz M, Villota J, Moneo I. Analysis of basophil activation by flow cytometry in pediatric house dust mite allergy. Pediatr Allergy Immunol. 2002;19:342–7. doi: 10.1111/j.1399-3038.2007.00656.x. [DOI] [PubMed] [Google Scholar]
- Bühring HJ, Streble A, Valent P. The basophil-specific ectoenzyme E-NPP3 (CD203c) as a marker for cell activation and allergy diagnosis. Int Arch Allergy Immunol. 2004;133:317–29. doi: 10.1159/000077351. [DOI] [PubMed] [Google Scholar]
- Kahlert H, Cromwell O, Fiebig H. Measurement of basophil-activating capacity of grass pollen allergens, allergoids and hypoallergenic recombinant derivatives by flow cytometry using anti-CD203c. Clin Exp Allergy. 2003;33:1266–72. doi: 10.1046/j.1365-2222.2003.01756.x. [DOI] [PubMed] [Google Scholar]
- Ocmant A, Mulier S, Hanssens L, Goldman M, Casimir G, Mascart F. Basophil activation tests for the diagnosis of food allergy in children. Clin Exp Allergy. 2009;39:1234–45. doi: 10.1111/j.1365-2222.2009.03292.x. [DOI] [PubMed] [Google Scholar]
- Tokuda R, Nagao M, Hiraguchi Y, Hosoki K, Matsuda T, Kouno K, Morita E, Fujisawa T. Antigen-induced expression of CD203c on basophils predicts IgE-mediated wheat allergy. Allergol Int. 2009;58:193–9. doi: 10.2332/allergolint.08-OA-0023. [DOI] [PubMed] [Google Scholar]
- Host A, Halken S. A prospective study of cow milk allergy in Danish infants during the first 3 years of life. Clinical course in relation to clinical and immunological type of hypersensitivity reaction. Allergy. 1990;45:587–96. doi: 10.1111/j.1398-9995.1990.tb00944.x. [DOI] [PubMed] [Google Scholar]
- Boumiza R, Debard A, Monneret G. The basophil activation test by flow cytometry: recent developments in clinical studies, standardization and emerging perspectives. Clin Mol Allergy. 2005;3:9–14. doi: 10.1186/1476-7961-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michova A, Abugalia M, Ivanova T, Nikolov G, Taskov H, Petrunov B. Comparison of two flow cytometry methods for basophil degranulation in patients sensitized to grass pollen. Allergy. 2006;61:1078–83. doi: 10.1111/j.1398-9995.2006.01087.x. [DOI] [PubMed] [Google Scholar]
- Monneret G, Gutowski M, Bienvenu J. Detection of allergen-induced basophil activation by expression of CD63 antigen using a tricolour flow cytometric method. Clin Exp Allergy. 1999;115:393–6. doi: 10.1046/j.1365-2249.1999.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinet JP. The high-affinity IgE receptor (FcεRI): From physiology to pathology. Ann Rev Immunol. 1999;17:931–72. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- Bock SA. The natural history of food sensitivity. J Allergy Clin Immunol. 1982;69:173–7. doi: 10.1016/0091-6749(82)90096-3. [DOI] [PubMed] [Google Scholar]
- Sampson HA, Scanlon SM. Natural history of food hypersensitivity in children with atopic dermatitis. J Pediatr Olga Ciepiela. 1989;115:23–7. doi: 10.1016/s0022-3476(89)80323-3. [DOI] [PubMed] [Google Scholar]
- Rubio A, Vivinus-Nébot M, Bourrier T, Saggio B, Albertini M, Bernard A. Benefit of the basophil activation test in deciding when to reintroduce cow milk in allergic children. Allergy. 2010. DOI: 10.1111/j.1398-9995.2010.02432. [DOI] [PubMed]


