Abstract
Percutaneous nephrolithotomy is a very commonly done procedure for management of renal calculus disease. Establishing a good access is the first and probably the most crucial step of this procedure. A proper access is the gateway to success. However, this crucial step has the steepest learning curve for, in a fluoroscopy guided access, it involves visualizing a three dimensional anatomy on a two dimensional fluoroscopy screen. This review describes the anatomical basis of the renal access. It provides a literature review of all aspects of percutaneous renal access along with the advances that have taken place in this field over the years. The article describes a technique to determine the site of skin puncture, the angle and depth of puncture using a simple mathematical principle. It also reviews the common problems faced during the process of puncture and dilatation and describes the ways to overcome them. The aim of this article is to provide the reader a step by step guide for percutaneous renal access.
Keywords: Fluoroscopy, Percutaneous renal access, Percutaneous nephrolithotomy, Learning curve, Kidney
Core tip: This article is a review of the various fluoroscopic guided renal access techniques. It provides an in depth description of the technique with the aim that the urologist can have a step by step guide of the procedure. It gives an anatomical basis of percutaneous renal access and gives description of determining the skin site, angle and depth of puncture. It also describes the difficulties faced and incorporates suggestions to prevent and overcome them.
INTRODUCTION
Rupel and Brown first reported Percutaneous nephrolithotomy in 1941[1]. Goodwin et al[2] described percutaneous trocar nephrostomy in a hydronephrotic kidney in 1955. The technique gained popularity especially after the description by Fernstrom and Johansson in 1976[3-5]. The improvement in endourological equipment and the advancement in techniques resulted in percutaneous nephrolithotomy (PCNL) getting accepted as the gold standard for the treatment of patients with renal stones larger than 20 mm in diameter[6]. Its popularity and acceptance amongst urologists and patients is largely due to the fact that it is minimally invasive and is associated with low morbidity[7]. Initially the procedure was done only in prone position using fluoroscopy guidance. However, in the last couple of decades use of ultrasonography alone or along with fluoroscopy has been used for percutaneous renal access[8]. Various modifications in the position of patient have also been described to overcome some limitations and drawbacks of the percutaneous renal access in prone position[9]. Despite these changes fluoroscopy guided access in prone position is still the most commonly used technique for PCNL[10]. The prone position is associated with a significantly shorter nephrostomy tract length and more potential access sites, which may improve ease and safety of percutaneous renal access[11]. In European countries, urologist establishes their own percutaneous renal access, but in the United States, access is often performed by interventional radiologists. Studies have shown lower stone free rate and a higher complication rate in radiologist performed renal access[12-14]. Despite these facts and the documented safety and efficacy of urologist acquired percutaneous renal access, as few as 11% of urologists who perform PCNL achieve access[15]. This low success rate is attributed to probably a lack of skill[16]. This is probably due to the difficulty in visualizing and mentally imbibing the three dimensional anatomy of the pelvicalyceal system on the two dimensional fluoroscopy screen[17]. The purpose of this review is to describe the various aspects of the technique of fluoroscopy guided percutaneous renal access in prone position. It is the attempt of the authors to provide a step by step guide of all aspects of the technique. Finally, the authors describe their technique in detail and the rationale behind it.
INSERTION OF URETERIC CATHETER
At the beginning of the procedure, a 5 Fr or 6 Fr ureteric catheter is inserted in the collecting system either using a rigid cystoscope (with the patient in lithotomy position) or a flexible cystoscope (with the patient prone). This is used to instill contrast to opacify the system. Also it can be used to flush saline so as to distend the system, flush small gravels during the process of stone fragmentation and at times to pass a glide wire for insertion of a double J (DJ) stent, at the end of the procedure. A Foley catheter is also passed by the side of the ureteric catheter. Both the catheters are secured with each other to prevent inadvertent slipping out of the ureteric catheter.
PRONE POSITIONING
This is an important maneuver which, if not done properly, can result in potentially serious injury to the patient. The ideal way would be to have the patient supine, have a separate trolley by the side of the operating table, shift the patient in supine position to the side trolley, remove the monitoring devices and then make the patient prone on the operating table and immediately connect the devices which have been disengaged or removed. These maneuvers should be done with adequate staff and with proper co-ordination between the anesthesiologist managing the airway, endotracheal tube and neck and the staff managing the chest and torso. Although cervical spine injury during prone positioning under anesthesia is rare, it has been reported with both over flexion and over extension during prolonged procedures[18]. Patients with cervical spine pathology, Down’s syndrome or rheumatoid arthritis or patients with myelopathic syndromes are at the greatest risk. Post operative visual loss is an uncommon (0.2% of spinal surgeries in one review) but grave complication of prone surgery[19]. The ability to maintain good ventilation along with the hemodynamic stability throughout the procedure is the challenge which the anesthesiologists face. For a healthy, adequately anesthetized patient, these may be clinically insignificant; however, for those with associated co morbidities, it can be very precarious. Hence, irrespective of whether the procedure is done under general or regional anesthesia, constant vigilant monitoring during the procedure is a must. Anesthesia for PCNL cannot and should not be taken lightly.
POSITION OF PATIENT
Care should be taken to ensure that the pressure points are properly padded and limbs are positioned in a way that undue stretch, especially of the joints is avoided. This would prevent inadvertent injury and stretch of the nerves. The chest and abdomen is supported in a way to ensure free movements for the pulmonary capacity is greater in the prone compared to the supine position[18]. After positioning the flank is properly prepared and the unsterile areas covered with drapes (Figure 1).
Figure 1.
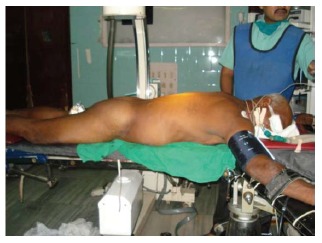
Position of patient.
ARRANGEMENT OF TROLLEYS
This is as shown in the figure. This helps to have a clear view of the fluoroscopy and endo camera monitors (Figure 2).
Figure 2.
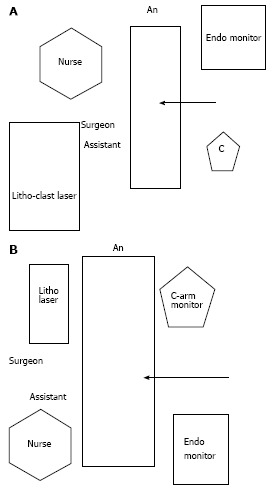
Arrangement of trolleys. A: Arrangement for lower pole puncture; B: Arrangement for upper pole puncture.
INSTILLATION OF CONTRAST
Contrast is instilled via the ureteric catheter to opacify the pelvicalyceal system and identify the calyx which should be punctured. The contrast should be diluted in ratio of 1:3. The ureteric catheter, if placed in the upper pole, should be pulled down a bit so that it is in the pelvis. This helps in proper filling of all the calyces. The contrast should be instilled slowly to prevent extravasation. There should be continuous fluoroscopy monitoring so that which calyces are filled earlier and which later can be seen and this helps to identify the posterior calyx.
WHICH POLE TO PUNCTURE?
The creation of a proper percutaneous renal access is the gateway to success or disaster in PCNL[10,20,21]. A basic understanding of anatomy is needed to plan this. The kidneys lie on the posterior abdominal wall against the psoas muscle with their longitudinal axis parallel to the oblique course of the psoas at an angle of 13° to 30° to the midline. Also, as the psoas major muscle is cone shaped, the kidneys, in their longitudinal axis have a dorsal tilt with the superior poles being more medial and more posterior than the inferior poles. As the hilar region is rotated anteriorly on the psoas muscle, the kidneys are rotated about 30° posteriorly and hence the lateral aspect of the kidney is posterior to the medial aspect. The kidneys are also angled 30°-50° behind the frontal (coronal) plane with the lower pole anterior to the upper pole[22]. In prone position, the pelvis tends to fall anteriorly on the psoas muscle; hence the lower pole, pelvis and the proximal end of the ureter are placed more anteriorly than the upper pole[23,24]. The calyceal drainage of poles of the kidneys is also very important. Sampaio found that, in the cases he studied, the superior pole was drained by only one midline calyceal infundibulum in 98.6% of cases; the inferior pole was drained by paired calyces arranged in two rows in 58% and by a single mid line calyceal infundibulum in 42% of cases and the mid pole was drained by paired calyces arranged in two rows (anterior and posterior) in 96% of cases[22]. This has important implications for percutaneous renal access as it will be easier to access endoscopically a polar region drained by a single infundibulum, which usually has suitable diameter, rather than a polar region drained by paired calyces. He also found that for best access to the pelvic-ureteric junction (PUJ) one should choose a pole whose calyx forms an angle of 90° or more with the PUJ[22].
The planning for puncture begins preoperatively by proper assessment of the imaging studies. Traditionally intravenous urography was used for functional and anatomical assessment of the collecting system. Nowadays, CT urography with coronal reconstruction is getting popular[25]. The advantages of the CT scan over intravenous urography is the ability to assess the spatial relationship of the kidney relative to the stone, depict the calyceal anatomy in 3D format to choose the access site, assess risk of pleural or bowel injury and even predict success of a sub costal fluoroscopic access for upper pole puncture. The preoperative detection of heptaosplenomegaly or the presence of retro renal colon allows serious complications related to tract placement to be avoided[26-29]. These advantages are offset by the slightly higher cost and lack of widespread availability of multiplanar CT scans in developing countries.
Whichever imaging modality is used the urologist has to select a pole for puncture which provides the straightest path along the stone axis and would provide maximum or complete stone clearance. A useful adjunct to make this decision would be to make an “outline-o-gram”. As shown in Figure 3A there is a complete stag horn calculus. The “outline-o-gram” as shown in Figure 3B indicates that most of the calculus can be cleared by a lower pole puncture. The mid pole calculus would need a separate puncture or use of a flexible nephroscope. Thus an “outline-o-gram” can serve as a guide to determine which pole to puncture and also to decide whether multiple tracts will be needed.
Figure 3.
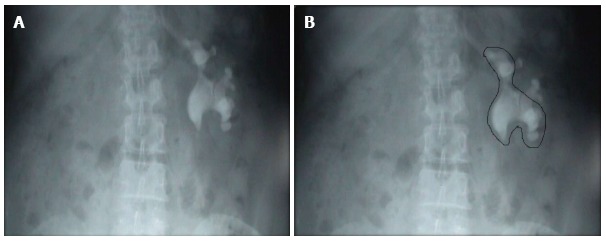
Outline-o-gram. A: KUB showing a Left staghorn calculus; B: Outline-o-gram suggests that most of the calculus can be cleared by the lower puncture and a separate puncture may be needed for the residual fragment.
WHICH CALYX TO PUNCTURE?
The literature is clear about the fact that it should always be the posterior calyx which should be punctured for a safe and complication free access[20,21].
WHY TO PUNCTURE THE POSTERIOR CALYX?
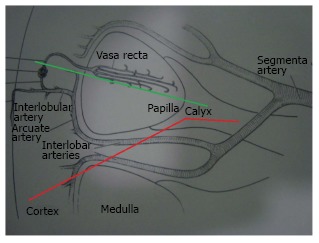
A good understanding of the renal anatomy provides answer to this question. The kidney gets its blood supply from the renal artery which divides into the anterior and posterior branch. These further divide into segmental arteries which supply specific areas of the kidney as shown in the figure (Figure 4). As these arteries are end arteries, there is a zone of relative avascularity between these two divisions, called as the Brodel’s line of bloodless incision. The potential for bleeding complications is the least in this area. Due to the renal rotation, the posterior calices are usually oriented with their long axis pointing towards the Brodel’s line. Hence puncture of a posterior calyx will traverse this relatively avascular zone[30]. Also, as the patient is prone, it will provide the direct path to the renal pelvis. If an anterior calyx is punctured, there is increased risk of bleeding as it does not traverse through the Brodel’s line. More parenchyma is traversed to reach the calyx, resulting in more renal damage. Also, as there will be an acute angle between the line of puncture and the infundibulum, entry in the renal pelvis will be difficult, associated with more torque and thus increased bleeding and damage to the renal parenchyma[20,21].
Figure 4.
Arterial blood supply of kidney.
CALYCEAL ORIENTATION-WHICH CALYX IS THE POSTERIOR CALYX?
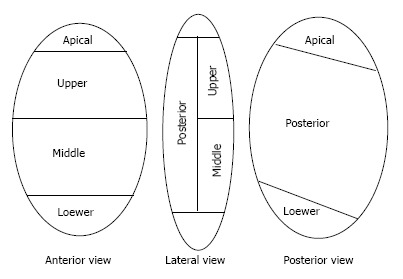
The renal papillae drain into the minor calyces which may be simple or compound. There are three drainage zones, upper, middle and the lower pole. Compound calyces are the rule in upper pole, are common in the lower pole and are rare in the middle pole.
Investigators have attempted to differentiate calyces as anterior or posterior solely on the basis of their medial or lateral orientation as seen on IVU. The available anatomical references on this aspect are contradictory, confusing and incomplete. In 1901, Brodel studied corrosion casts of 70 cadaveric kidneys. He depicted the anterior calyces as medial and posterior as lateral[31]. Hodson, in 1972, described exactly the opposite, i.e., the anterior calyces located laterally and posterior calyces located medially[32]. Then, in 1984, Kaye and Reinke[33] measured calyceal angles from the axial CT images. They concluded that the Brodel pattern is seen in 69% of right kidneys while 70% of left kidneys have a Hodson pattern[33]. Sampaio et al[34,35] studied 140 endocasts and found that the anterior calyces are lateral in 28%, posterior calyces are lateral in 19%, and in 53% endocasts the anterior and posterior calyces had varied positions, superimposed or alternately distributed (in one region the most lateral were the anterior calyces and in another the posterior calyces)[34,35]. He found that the calyceal orientation was region dependent. The typical anterior and posterior pattern of the calyces is seen only in the middle pole[22]. The lower pole has this arrangement in only 58% cases while the upper pole almost uniformly has a compound calyceal system[22]. This implies that in the lower and upper pole the calyces are dominantly oriented in the direction of their respective poles. This has been further studied by 3D CT renderings which have also looked at the primary plane of the calyceal group. Miller et al[17] found that in the upper pole the primary plane of the calyces in the upper pole was Medial/Lateral and generally neutral relative to the anteroposterior axis of the kidney. As the upper pole is more posterior in the prone position, access via any calyx would provide a working tract that parallels the longitudinal axis of the kidney. This would mean access via an angle which would allow rigid instruments to reach most of the calyces in the kidney[17]. However, preferably the lateral most calyx should be punctured in the upper pole as puncturing a medial calyx is associated with significant risk of causing injury to the posterior segmental artery[36]. Eisner et al[37] studied the lower pole anatomy by CT scans in 101 units. They found that if there were two calices in the lower pole, the medial calyx was anterior in 95% of units while the lateral calyx was posterior in 93% of units. If there were 3 calices in the lower pole, than the medial most calyx was anterior in 93 of units. In such renal units the lateral to most medial, i.e., the second calyx was posterior in 70% of units while the lateral most calyx was anterior in 71% of units. In 31% of cases, no calyx was truly posterior. In these kidneys, though both the calices were anterior, one of the calices was less anterior than the other. Their study showed that regardless of the number of lower pole calices, the most medial calyx on two dimensional imaging is anteriorly facing 94% of times. They recommended that the calyx, just lateral to the medial calyx, the second calyx, is statistically the most likely to be posterior facing and the most posterior positioned calyx[37].
HOW TO IDENTIFY THE POSTERIOR CALYX ON FLUOROSCOPY?
On account of the unreliability of the antero-posterior radiography to determine the optimal posterior calyx for entry additional maneuvers are needed[35]. With the patient in prone position, diluted contrast when instilled will fill the dependent anterior calices first. Thus the posterior calices will be filled later and would appear less dense[21]. Injection of 5-10 mL of air via the ureteric catheter also helps to identify the posterior calices as air will preferentially enter these calices when the patient is prone[21,38]. Despite these maneuvers if there is dilemma in indentifying the posterior calyx, movement of the C arm can help to identify the posterior calyx. In the prone position, the posterior calyces move in the opposite direction to the image intensifier on the C arm. If the C arm is rotated towards the surgeon then the posterior calices move away and shorten. Vice versa, if the C arm is rotated away from the surgeon then the posterior calices appear elongated. Thus by moving the C arm way from the surgeon one can identify the laterally placed calices as posterior and by moving the C arm towards the surgeon the posterior calices appear more medially placed and appear end on[9].
NEEDLE USED FOR PUNCTURE
A diamond tip needle and not a bevel tip needle should be used for puncture. A diamond tip needle has symmetrical tip which exerts equal force in all directions on the tissue. Hence the tissue is cut in the moving direction of the needle tip. A bevel-tip needle exerts forces asymmetrically so cutting of the tissue occurs at an offset angle depending on the bevel angle, needle flexibility and tissue properties[39]. The size of the needle used for puncture is a matter of debate. The options are a 21 gauge needle (which allows a 0.018 inch guide wire) or an 18 gauge needle (which allows a 0.035 inch guide wire). The 18 gauge needle is stiffer but more traumatic. The 21 gauge needle is less traumatic but less stiff and hence cannot maintain the trajectory adequately. Also, the 0.018 inch guide wire that passes through the 21 gauge needle must be exchanged for a standard 0.035 inch guide wire for subsequent tract dilatation. This requires an extra step, which adds to the complexity of the procedure and increases the risk of loss of access. Weighing the pros and cons of both it would be rational to use the 21 gauge needle when the surgeon is less experienced or if minimizing trauma is the need of the moment. The 18 gauge needle should be used by an experienced surgeon who is confident of attaining access with minimum attempts[40].
WHAT SHOULD BE THE TRAJECTORY OF THE NEEDLE?
Renal pelvis should not be punctured directly as there is very high risk of injuring a retro pelvic vessel (artery and/or vein). Studies by Sampaio have proved beyond doubt that puncture through the infundibulum of a calyx is associated with a significant risk of significant bleeding from interlobar vessels. There is an added risk of through and through puncture of the collecting system. The risk of injury to a major arterial vessel is maximum in the upper pole where puncture of the upper pole infundibulum puncture may cause damage to posterior segmental artery, which is related to the posterior surface of upper pole infundibulum in 57% of cases. Damage to this artery may lead to loss of upto 50% of the renal parenchyma as well as serious hemorrhage[30,35].
The trajectory of the needle during puncture should be such that it aims at the fornix and not at the infundibula (Figure 5). In other words we should aim for the center of the calyx posterolaterally via the renal parenchyma. When puncture is made through a fornix, no arterial injury occurs and venous injury occurs in less than 8% cases[30].
Figure 5.
Trajectory of needle during puncture.
CRITERIA FOR A GOOD PUNCTURE
Percutaneous renal access through a calyx must meet five conditions that guarantee safe access and avoids complications[41]: (1) Access should be performed from a posterolateral aspect; (2) Access should be through the renal parenchyma; (3) Access should be towards the center of a calyx posterolaterally; (4) Access should be towards the center of the renal pelvis and as a result of these four conditions; and (5) The trajectory does not damage any major blood vessels.
TYPES OF FLUOROSCOPY GUIDED PUNCTURE
There are two types of fluoroscopy guided puncture techniques-the Bull’s eye and the Triangulation Technique. Besides these two there are a number of variations described[42].
BULL’S EYE TECHNIQUE
It is also called the Eye of the Needle technique[20]. The target calyx is identified with the C arm at 0° in the axial plane. Then the C arm is rotated 30° towards the surgeon and the calyx to be punctured would appear end on the fluoroscopy screen. A tilt of 5°-10° in the caudal direction for the lower pole or in the cranial direction for the upper pole may be necessitated to have a circular end on appearance of the target posterior calyx[21]. The position on the skin overlying the selected calyx is then marked and the puncture initiated. The needle is advanced at the end of full-expiration. It is seen as a Bull’s eye (as a dot) on the fluoroscopy screen. If a longitudinal segment of needle is seen then it indicates that the trajectory is not correct and adjustment needs to be made accordingly. The C arm may be rotated by few degrees away from the surgeon to get a proper perspective of the depth of the puncture. The needle will now be seen in profile. It is then advanced to puncture the calyx. Free efflux of urine confirms the position in the collecting system[20,21,41,42]. To minimize radiation to the hands, the needle could be held with hemostat, sponge forceps or a purpose-built radiolucent needle holder (Figure 6).
Figure 6.
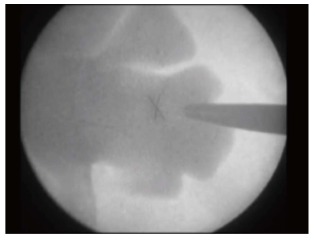
Bull’s eye appearance of the needle.
Modifications of the bull's eye technique
Bilen et al[43], described the use of an in-line laser pointer to guide renal access in which the laser was attached within the field of the receiving head of the C-arm fluoroscopy unit. Ko et al[44] described a further modification using a C arm mounted laser positioning device where the laser beam is focused on the hub of the needle continuously so that the correct alignment is maintained during the puncture without the use of fluoroscopy. This approach may reduce fluoroscopic exposure early in the learning curve. As experience increased the muscle memory leads to maintenance of the correct needle alignment[42].
TRIANGULATION TECHNIQUE
Triangulation technique is the technique of using two known points of reference to locate a third unknown point. It is guided by biplanar fluoroscopy. The medial and lateral plane is assessed with the C arm at 0°. The depth is assessed by rotating the C arm in the cranial or caudal direction by 30°. The target calyx is identified with the C arm at 0°. Then the line of puncture is aligned with the infundibulum. With the C arm at 0° the needle is introduced through the skin incision. The left and right, i.e., the mediolateral adjustments are made and the needle is aligned with calyx. Then the C arm is rotated 30°, towards the head end for lower pole punctures and towards the foot end for upper pole punctures. The needle is then oriented in the up and down, i.e., the cephalo-caudal position so that the orientation is again towards the desired calyx. When making the adjustments in one plane it is necessary to maintain the orientation of the needle in the other plane. The needle is then advanced with the C arm tilted 30° to give an idea regarding the depth and the respiration suspended at end expiration. After advancing the needle for several centimeters shift the C arm to 0° to see that the trajectory of the needle is still properly aligned to the target calyx in the mediolateral plane. If necessary the needle trajectory can be readjusted to maintain proper targeting. It is imperative that to minimize trauma to the renal parenchyma, the adjustment of the needle plane should be done when the needle is outside the renal capsule and not when the needle is in the parenchyma. A slight jiggle of the needle causing indentation of the desired calyx is a further sign that the trajectory of the needle is correct. If the needle position in the medial-lateral and cephalo-caudal planes is maintained, the needle should enter the targeted calyx[20,24,39-41]. It is preferable to use the 18-gauge rather than a 21-gauge needle with the triangulation technique, as its stiffness provides better stability to help maintain angle of entry[39].
Comparison of Bull’s eye and Triangulation technique
In the Triangulation technique, the puncture is along the stone axis, i.e, in alignment with the infundibulum. This decreases the need for excessive torque on the renal parenchyma by the rigid instruments, which may cause renal trauma and bleeding[20]. Tepeler et al[10] did a comparison of the Bulls’ eye and the Triangulation techniques and found no difference between the two as regards operation time, fluoroscopy screening time, duration of hospitalization and blood transfusion rate. They found a slightly greater drop in hematocrit and complication rate in the group undergoing access by the Bull’s eye technique as compared to the Triangulation technique. However, the difference was not statistically different.
The advantage of the triangulation technique over the eye-of-the-needle” technique is that the needle cannot be passed too deeply because the depth of advancement is monitored continuously[39]. Also, the triangulation technique alone fulfills the five criteria of a successful puncture[41]. The disadvantage of the triangulation technique is that maintaining both the medial-lateral and cephalo-caudal planes are difficult because both are not being monitored at the same time as in the “eye-of-the-needle” technique. Complex visual spatial skills are required in performing this task when using a C-arm fluoroscopy unit, especially by the novice surgeon[44]. It is at this juncture that multiple attempts are needed by the urologists and excessive use of fluoroscopy occurs especially by a beginner. This is also the aspect which has the steepest learning curve for an urologist getting trained in percutaneous nephrolithotomy[45]. Usually, during the learning curve the problem comes in the assessment of depth with the C arm in the oblique position. Whether the needle is superficial or deep to the calyx has to be ascertained by the surgeon and adjustments made accordingly[46]. The easiest way to determine this would be to place another needle on the skin surface over the target calyx. If the calyx is between the two needles than the puncture needle is deep and should be adjusted superficially. If the target calyx is below the two needles, then the puncture needle is superficial and should be adjusted towards the depth.
Modifications of triangulation technique
Several new approaches and refinements have been described to improve access and reduce the learning curve of the surgeon. Mues et al[47] described a geometric model to create a plane of coincidence between the C arm and the needle, each at the same angle of 20°-30° from the targeted calyx, but in opposite directions. For lower pole access, the C arm is rotated cranially 30° from the vertical plane and a needle is advanced from a position distal to the calyx, rotated caudally 30° from the vertical plane. For mid pole and upper pole calyceal access the C arm is rotated 20° away from the surgeon and the needle is advanced from a position lateral to the calyx at an angle of 20° towards the surgeon from the vertical plane. During the procedure the C arm remains fixed and the needle is advanced till the point of coincidence between the calyx and the needle tip is reached. This technique avoids the need for C arm manipulation and thus potentially reduces time need to achieve the puncture[41]. This technique, however, requires a plumb, protractor and ruler to calculate, and confirm the necessary measurements. Also, it presumes that the angle of convergence would be 30° at the lower pole and 20° at the other poles. Considering the wide variations in the structure of the kidney that occurs with the varying degrees of hydronephrosis that occurs, this may not necessarily be always true. Liatsikos et al[48] described a technique to take advantage of the ability of triangulation technique to target a calyx from a preselected puncture site. After clearing the calculus from the initial puncture site, the sheath is withdrawn and additional calices which need to be approached for complete stone clearance are punctured from the initial puncture site. A single nephrostomy tube can be left despite multiple entries through the single incision[42,48]. The tracts are from a single site but in different directions. This does not reduce the chance of complications. Moreover, attempt of maneuvering the rigid nephroscope may increase the torque on the renal parenchyma with resultant increase in bleeding. Mozer et al[49] have described a computer generated system which can be used to project the ultrasound nephrostomy tract onto fluoroscopic images virtually. The surgeon, thus, has the benefit of having preview to a three dimensional anatomy while doing the procedure instead of the usual two dimensional fluoroscopy picture. Though exciting, it requires a special system which is not routinely available. Newer C arms utilizing softwares to provide a three dimensional picture have also been used to obtain renal access in animal models[50]. Robot assistance for fluoroscopic percutaneous renal access has also been studied and is under evaluation[51,52]. These futuristic techniques have not gained widespread acceptance A study comparing robot assisted renal access with standard manual access showed that though the mean number of access attempts was comparable, the robot took lesser time to achieve puncture (10.4 min vs 15.1 min). However, conversion to manual access was needed in 3 cases where the robot was unsuccessful[53]. A stereotactic localization system with specially designed instruments have been described by Li et al[54]. It uses the Pythagoras principle of right angle triangle to calculate the depth of puncture. Then using specifically designed and patented instruments the puncture is made with precalculated depth and angle of puncture. The same instrument is then used for dilatation of the tract. The authors found that their technique is associated with higher efficiency, better stone clearance and lower morbidity, which they attribute to the greater accuracy of the puncture. This technique was not found to be useful when the puncture angle was less than 30°, because the buttocks of the operator would be in the way. Also the authors generally selected the puncture point with the same distance vertically and horizontally, which means at 45° from the skin to the stone. This again makes the principle of puncturing quite rigid as the variations in the pelvicalyceal anatomy may preclude adherence to such rigid principles. Recently, Hatipoglu et al[55] described a monoplanar access technique. The chosen calyx is marked with a clamp. For the lower pole puncture the needle is placed 1 cm below and medial to the 12th rib. The needle is placed with a 30° angle to the sagittal plane and is directed toward the desired calyx. If puncture fails, the needle is retracted approximately 1 cm intracorporeally, and its angle of entry adjusted on the same vertical plane and reinserted. For the mid and upper pole puncture the needle is held perpendicular to the spinal column and at a nearly 30° to the horizontal plane to access targeted middle and upper calyces to reach the pelvis and lower poles. The authors proposed that as the C arm is fixed in an anteroposterior position and thus rotation is avoided, the operative time needed for puncture is less[55]. However, there were no upper pole punctures in this study. In the initial learning curve a surgeon would find this technique difficult to master. Also, using a pre fixed puncture point on the skin for all lower pole punctures and directing the needle at a fixed angle of 30° to the sagittal plane may not always be a correct approach especially considering the variation in the position and direction of the lower pole calyces.
Hybrid technique
Rationale thinking suggests that the three most important things needed to achieve a successful percutaneous renal puncture are the site of skin entry, the angle of entry and the depth at which the puncture is achieved. Determining the correct point of skin puncture is important in the triangulation technique because a skin puncture that is too medial or lateral to the desired optimum point of entry would result in a tract of variable length and angle of entry in the calyx. This would interfere with proper access and would cause excessive torque on the parenchyma during maneuvering of the rigid nephroscope in the pelvicalyceal system. The literature does provide guidelines when it comes to determining the site of skin puncture. To avoid injury to the colon, the puncture should be medial to the posterior axillary line but not too medial as it would traverse the paraspinal muscle causing increased postoperative pain and would probably be directly on the renal pelvis without traversing the renal parenchyma. The puncture that is too close to the rib may injure the intercostal nerve and vessels and hence is to be avoided. For lower-pole access, the skin puncture should be 1 cm inferior and 1 cm medial to the tipoff the 12th rib[20,55-57].
We have described the technique of determining the site of skin puncture, which amalgamates the advantages of both the bull’s eye and triangulation technique and hence is called as the hybrid technique[41,57]. With the C arm at 0°, the site of skin corresponding to the target calyx is marked as point A. The C arm is then rotated 30° towards the surgeon. The point on the skin corresponding to the target calyx and forming a bull’s eye with the needle is marked as point B. In The Bull’s eye technique we take a puncture at the point B. However in the Triangulation technique, the puncture is along the stone axis in alignment with the infundibulum. If we take the target calyx as the center of a sphere, then we have an imaginary circle on the skin where the point A is the center of the circle. The distance from point A to B will be the radius of the circle. The radius remains the same irrespective of the direction in which it is measured from the center of the circle. Thus, when we take a line along the stone axis where we intend to take a puncture-the site of skin puncture is marked using this principle. This means that the point B1 is marked on the skin such that the distance from point A to B1 is equal to the distance between A to B, i.e., the radius of a circle with the target calyx being its center. This is how we determine site of skin puncture in triangulation technique[57] (Figures 7 and 8).
Figure 7.
Hybrid technique. Point C is the calyx to be punctured. Point A corresponds to the Point C with the C arm at 0°. Point B corresponds to the point C with the Carm rotated towards the surgeon by 30°. The needle held at Point A or B is seen as a Bull's eye effect on the Carm monitor. The distance between points A and B is measured.
Figure 8.
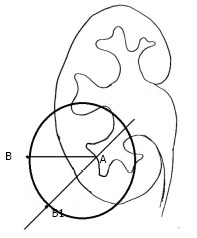
Hybrid technique. “A” is the point on the skin which corresponds to the targeted calyx with the C arm at 0 degree and is the center of an imaginary circle. The distance between “A” to “B1” is equal to the distance between the “A” to “B”, i.e., the radius of the circle.
Once the site of puncture is determined the next critical step is to access the centre of a posterior calyx with the needle directed at an appropriate angle. This step of hitting the calyx at the depth often requires maneuvering the C arm in different directions, either towards the surgeon (in bull’s eye technique) or in an oblique cephalo-caudal direction (for the triangulation technique) and requires understanding a three dimensional anatomy on a two dimensional fluoroscopy monitor. This step requires maintenance of the needle in one plane while making the adjustments in the other plane and not surprisingly, multiple attempts are needed and excessive use of fluoroscopy occurs, at this step, especially by a beginner[20,45]. Maintenance of needle orientation in one plane while making the adjustment in the other plane is critical for a proper puncture. This is also the aspect which has the steepest learning curve for an urologist getting trained in percutaneous nephrolithotomy[45].
We describe our technique of using a simple mathematical principle to determine the angle and depth of puncture in fluoroscopy guided percutaneous renal access in prone position. We have used it in > 150 cases for lower, mid and upper pole punctures with > 95% success in first attempt and no pleural, visceral or hemorrhagic complications. This has recently been accepted for publication.
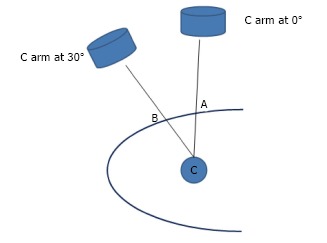
In the Bull’s eye technique, the angle at which the needle is seen as a dot is the angle at which the puncture is made. Our Hybrid technique utilizes this principle. With the needle at point B and the C arm rotated 30° towards the surgeon and the needle forming the Bull’s eye; the angle that the needle makes with the skin surface is measured using a protractor (Figure 9). One needs to take care that the protractor is held parallel to the operating table. Using the principle of sphere and circle as described earlier; if we are hitting the calyx by using the triangulation technique from the point B1-the angle of puncture would be the same with probably variations of 1-2 degrees due to the not so perfectly flat contours of the body surface. The third component of the hybrid technique is to determine the depth of puncture. What we have till now is an imaginary triangle (Figure 9) where we know: (1) One side - the distance between point A to B which is marked on the skin; (2) One angle - which is 90° with the C arm at 0°; and (3) Another angle- which is measured using the protractor at the point B.
Figure 9.
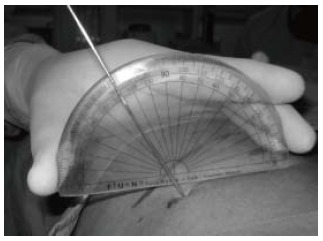
Hybrid technique. The angle which the needle makes with the skin surface is measured using a protractor.
With this information; by using the Universal triangle solver application from Google play store we can determine the depth. In this application, if we put the two angles and one side then, by the law of sines, it calculates the other two sides and the angle. For example, if the distance AB is 4 cm and the angle calculated by the protractor is 65° and with the other angle always being 90°- by universal triangle solver-the depth will be 9.5 cm.
The same principle can be applied in the triangulation technique. The C arm is brought to 0°. The line of puncture is determined in alignment with infundibulum from point A. On this line the point B1 is marked. From the Point B1 access can be obtained using the triangulation technique (Figure 8). The angle of puncture is as determined by the protractor earlier using the bull’s eye principle. The depth of puncture is the same as calculated earlier. As the angle of entry is known and the depth pre calculated, the needle is advanced with the C arm 0° position only (without the need to take it in oblique position) and the puncture is made.
In the technique described by us we have assumed the target calyx as the centre of a sphere. If we have to hit the centre of a sphere from the surface, the distance traversed from any point on the surface to the centre would be the same. Hence once we have marked the point B on the skin surface using the bull’s eye technique and then mark point B1 for the skin entry using the triangulation technique; then the distance from C to B or from C to B1 will be the same, i.e., the radius of the sphere. Also the angle of entry from B or from B1 towards point C would be nearly the same, with only minor difference, because of the not so perfectly flat contours of the body.
But, this minimal difference would not cause any major hindrance in achieving access by the technique described because the angle of puncture and thus the trajectory of the needle would not have much variation. This was seen by us in our study. The difference between the calculated and the actual depth ranged from 0-3 mm. Also, as the angle of entry is known the fluoroscopy screening time and the time needed to achieve puncture decreases as multiple movements of the C arm are not required.
The technique described by us is applicable for both the bull’s eye and the triangulation method. It describes the three most important things needed to achieve a successful percutaneous renal puncture: the site of skin entry, the angle of entry and the depth at which the puncture is achieved. It relies on simple tools. There could be some errors which could creep in especially if the protractor is not held parallel to the operating table, but this could be overcome easily with minimal experience (and the assistant telling that the protractor is parallel to the table or not). But so far this technique has not been compared with other techniques. The applicability and validation of this technique in the hands of others is yet to be ascertained. This would need a controlled prospective study involving many surgeons of equal experience and comparison with the traditional technique. It would then ascertain whether this technique is associated with a lesser fluoroscopy time, more accuracy and lesser learning curve as proposed by us. The grade of hydronephrosis can affect the puncture with the access being relatively easier for higher grades of hydronephrosis. However, if the angle, i.e., the trajectory of puncture is correct, as described by this technique, the puncture would be easier and precise even in lesser grades of hydronephrosis.
CONFIRMATION OF PUNCTURE OF POSTERIOR CALYX
If air has been instilled during opacification of the pelvi calyceal system, air will be aspirated followed by a free flow saline especially if it is instilled through the ureteric catheter. After this when the glide wire is passed, while maintaining the angle of the needle, in enters the pelvis easily. No manipulation is needed. On the contrary if the anterior calyx has been punctured than the glide wire will be coiled in the calyx, will not enter the pelvis easily or will do so only after much manipulation (Figure 10).
Figure 10.
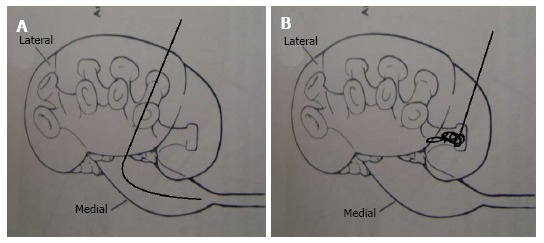
Calyceal puncture. A: If the posterior calyx is punctured than the glide wire passes easily in the pelvis; B: if the anterior calyx is punctured than the glide wire gets coiled in the calyx before it makes its way to the pelvis.
PASSAGE OF GLIDE/GUIDE WIRE
If a 21G needle has been used for puncture than a 0.018 inch guide wire is passed initially which has to be exchanged later for a 0.035 inch guide wire. If a 18 G needle has been used than 0.035 inch glide wire can be passed. Initially a J tip Teflon coated guide wire was used. Nowadays the use of an angled tip hydrophilic glide wire is increasing. The maneuverability, resistance to kinking and ease with which it can be negotiated in the ureter across an impacted calculus or be coiled in a distant calyx are the distant benefits of this wire. But the slippery nature of the hydrophilic wire makes it prone to displacement. Hence, it should be replaced with a stiffer wire such as a 0.035 inch Zebra or an Amplatz super stiff guide wire[21,41]. Another caution which needs to be exercised in passing the hydrophilic glide wire is to keep it wet. The dried tip can be stiff and cause inadvertent perforation of the collecting system.
INCISING SKIN AND FASCIA
The skin should be incised adequately so that the dilators and desired size of Amplatz sheath can be introduced easily. The correct way of incising the fascia would be to use the knife along the needle under fluoroscopic guidance as a lumbotome. The fascia should be incised in two planes at right angles to each other. This is especially important in patients who have scarring as a result of previous surgery. One may use an 18 G coaxial fascial incising needle (Cook) taking care to avoid lacerating the nearby sub costal or intercostal neurovascular bundle on the inferior rib margin[21].
GUIDE WIRE AND SAFETY WIRE
Traditional teaching gave much emphasis on the placement of a safety guide wire to access the tract in the event of the inadvertent slipping out of the working guide wire[20]. Many surgeons nowadays do not find it necessary to place a second safety wire especially if the guide wire is passed all the way in the bladder and more so it is a super stiff guide wire[21]. However during the learning curve it is prudent to have a safety wire. This can be introduced alongside the initial wire using a dual lumen catheter or 8/10Fr coaxial dilator of the dilatation canula (Karl Storz)[20,21,41]. Dilatation should be done over the stiff wire and not over the slippery hydrophilic wire.
TRACK DILATATION
The dilatation of the tract for creation of the nephrostomy access is an integral step of PCNL. Tract dilatation is performed to increase the size of the percutaneous wire access so that working instruments can be inserted in the pelvi-calyceal system (PCS). The size of the tract should be increased to 24 or 30 Fr size in most cases with the use of specialized dilators. The function of the dilator is to enlarge the tract in a noninvasive manner and to make renal access easier. The dilated tract is then maintained by placement of an Amplatz sheath.
Tract dilatation can be acute or chronic[58]. Chronic dilatation is done by placement of a percutaneous nephrostomy tube, which is gradually dilated over few days by sequentially replacing it by larger tubes. Acute dilatation is done just before the therapeutic procedure. The tract is dilated either by sequential (Amplatz dilators) or telescopic coaxial dilators (Alken dilators). These are rigid dilators. Balloon dilators are also in vogue and have results similar to rigid dilators. The chronic dilatation approach was once the conventional method by which renal access surgery such as PCNL were done; however, in recent years one stage acute dilatation method has become preferred due to its low risks of patient morbidity and decreased time, which allows less room for complications.
Alkens dilators
These are rigid metal dilators that are introduced over a central guide rod. Progressively enlarging coaxial stainless steel dilators help to dilate the tract from the 8 Fr guide rod up to 30 Fr. The guide rod has a round bulbous end prevents the sequential dilators from over-shooting. The advantages of the Alkens dilator system are that it is reusable, hence inexpensive and importantly is able to dilate even when there is dense perinephric scarring[59]. The disadvantage is that the same characteristics that make the Alkens dilator so effective are also the reasons why the rigid metal dilators can do considerable damage.
Amplatz dilators
These are semi rigid plastic dilators that are passed over an 8-Fr PTFE guiding catheter that fits over a 0.035-inch guide wire. They can also be passed over a guide rod. The dilators are passed one after the other, not coaxially like the rigid metal dilators but progressively, by advancing one dilator, removing it, advancing the next larger dilator, and so on until the final tract diameter is achieved. Finally, the working sheath is passed over the final dilator and then the dilator and 8-Fr catheter are removed, leaving the working wire and sheath in place. The dilators are made in increments of 2 Fr, but if the tissue being dilated is soft, then not every dilator needs to be used.
The advantages of Amplatz dilators are that trauma experienced by the collecting system is theoretically less probable than the trauma experienced by the collecting system using rigid metal dilators, but the disadvantage is that bleeding can happen each time a dilator is withdrawn. As these are disposable dilators, they are more expensive than the Alkens dilators.
There have been many comparative studies[59-63] between the two dilator systems but experienced urologists have found no difference between the two systems in terms of safety. Alkens dilators may be preferred in patients who have tight fitting staghorn calculi, as Amplatz dilators need some space in the calyx for dilatation. In calyces that have no space, the dilatation may remain short due to tapered end of the dilator.
Balloon dilators
Here a pressure balloon is used for rapid tract making[64]. The Amplatz sheath is back loaded on the balloon and is placed once the balloon is adequately distended. The balloon dilator is expensive and may be difficult to use in patients with densely scarred tissue. The dilators may have an advantage when operating on a hyper mobile kidney. As it is a single step dilatation that causes tamponade, the bleeding is expected to be less, but not all studies have documented less bleeding and transfusion as compared to the Alkens and Amplatz dilators[59,65-67].
In an effort to make tract making rapid, easy, and blood less, multiple single step techniques have been described. The simplest is using the largest Amplatz dilators without the initial smaller dilators. In difficult situations where scar tissue is present around the kidney, collings knife or plasma vaporization has been used for tract making[68]. Two new dilatation systems described have been a radially expanding single step dilator system[69] and the 5pang system[70]. Both the systems of the advantage of not removing the needle and hence the dilatation is over s rigid system resulting in less chances of kinking of guide wire. Also the dilatation would be faster. However, so far it has been a single center experience and multi center experience with these two systems has not been described.
The important principles of tract dilatation are: (1) A proper planning of the procedure and correct choice of the calyx of entry is vital for the success of PCNL. This needs a study the radiologic images prior to the procedure; (2) The tract should be dilated only till the minor calyx. If over-dilatation happens, it can traumatize the infundibulum, renal pelvis or uretero-pelvic junction. Trauma to the anterior wall of the PCS can cause significant bleeding that may be difficult to control. It is always better to under dilate than to over dilate and cause trauma; (3) The success of tract making is dependent on maintaining the angle, depth, and the direction of the dilatation; (4) Every step of dilatation should be monitored on fluoroscopy; (5) The collecting system should be kept distended during dilatation by instilling in the system either contrast or saline. This is instilled by the OR assistant through the ureteric catheter; and (6) Adequate lumbotomy is important for safe dilatation.
AMPLATZ SHEATH
The use of an Amplatz Sheath during percutaneous renal procedures has become standard. No matter the type of dilator used, rigid or balloon, or the technique of track dilation, one-step or multi-stepped, an Amplatz sheath is always used. The Amplatz sheath serves many purposes: (1) Amplatz sheath maintains the tract during procedure; (2) It causes tamponade of the tract and reduces bleeding. The beveled end of the Amplatz sheath can be used to tamponade a part of renal parenchyma that is actively bleeding[71]; (3) It protects the renal parenchyma from injury by the instruments used in renal procedures; (4) The use of Amplatz sheath maintains a low-pressure system and reduces fluid intravasation. Maintaining a low-pressure system would be important in patients with infected calculi as the risk of sepsis would reduce; and (5) Amplatz sheath helps in removal of calculi and prevents parenchymal injury by broken ragged stone edges.
WHEN TO DO MULTIPLE PUNCTURES?
The amount of bleeding, parenchymal damage, morbidity as well as the risk to the patient increases with increase in the number of punctures[72]. It is important to plan the first puncture in a way that multiple punctures are avoided. Use of flexible nephroscopy and flexible ureteroscopy would also reduce the need for multiple punctures[73].
Multiple accesses may be needed when treating large and complex stones and staghorn calculi. In this situation the first tract is made in a way that most stone bulk can be removed through it. The accessory tracts can be mini-PCNL tracts for peripheral small calculi. In this situation, upper calyx has an advantage as it affords a direct access to the upper calyx, renal pelvis, all components of the lower calyx and the upper ureter[74]. In selected situations where the calculi are smaller than the neck of the calyx, percutaneous calyceal lavage can be done to flush the calculi in the renal pelvis so that they can be picked up through the primary tract. If necessary, multiple tracts can be safely made in experienced hands with the intent of complete stone clearance[75].
In a complex situation, the plan of management would be as follows: Make the primary tract in a way to clear maximum stone bulk. If access to a flexible nephroscopy with holmium laser is available, use this to prevent additional tracts. If these facilities are not available, percutaneous calyceal lavage, mini-tracts or accessory tracts can be made.
HOW TO MAKE A SAFE SECOND TRACT?
The second or multiple tracts tend to bleed more than the primary tract because when the second tract is made, it is not possible to opacify the system. The puncture is more often directed to the stone and not to the calyx. Also the bleeding and fluid extravasation through the first tract can alter the anatomy. To prevent this, if a second tract is anticipated, it is better to place the guide wires in the calyces where the second tract is expected before the first tract is dilated. The advantage of a pre-placed guide wire is that the proper placement of the tract is possible but the disadvantage is that in some patients this wire may not need to be dilated. The advantages of a proper placed tract far outweigh the risk of a tract made aiming for the stones.
HOW TO MINIMIZE RADIATION?
The risk of radiation is quite high in patients with stone disease during their evaluation and treatment. Recently, two centers have studied the radiation dose in a patient with a primary acute stone event over a 1 year period. They found average radiation to which these patients were exposed was 29.7 mSv, and 20% of the patients were exposed to > 50 mSv[76]. This dose exceeds the International Commission on Radiological Protection recommendation on limits for occupational exposure to radiation, which is 20 mSv averaged over a 5-year period with not nore than > 50mSv in any single year. In comparison, a typical CT of the abdomen and pelvis without contrast exposes patients to a median of 15 mSv[77]. Fluoroscopy during percutaneous nephrolithotomy is associated with radiation exposure not only to the patient but also to the surgeon and the operation theatre staff[78]. High Body Mass Index, high stone burden, and increasing number of access tracts are associated with an increased radiation exposure. Branched stones and the presence of hydronephrosis are associated with decreased radiation exposure[79,80]. Proper planning of the procedure by an experienced surgeon is very important for reduction of radiation exposure[81]. It is the duty of the surgeon to reduce this health hazard for all concerned. Following steps can be taken to minimize radiation during PNL: (1) The surgeon and the staff must always wear radiation protection gowns, thyroid guards and radiation protection gloves[82]; (2) It is important to limit the time of exposure to minimum necessary. Using short bursts of fluoroscopy and using the “last image hold” feature of the fluoroscopy unit reduces radiation exposure[83]; (3) The image intensifier should be placed as close to the patient as possible, fluoroscopy beam should come from under the table, be focused on the area of interest and a pulsed fluoroscopy mode should be used[25]. The use of air instead of iodinated contrast may further reduce the radiation exposure[84]; (4) Keeping the fluoroscopy unit foot pedal with the surgeon and thinking during and after screening are other small precautions which can decrease the radiation; (5) For lower pole punctures using triangulation technique tilt the C arm cephalad and vice versa for upper pole puncture; and (6) Hold the needle in a way that the hands get minimal radiation exposure. Use of an instrument to achieve this would reduce radiation exposure. Needles and dilators that have distance marked on them can help in reducing radiation as the fluoroscopy can be used once the needle-tip is near the kidney[40,85].
PREVENTING VISCERAL INJURY DURING PCNL
Any abdominal organ close to the kidney can be injured during percutaneous renal surgery including the colon, duodenum, jejunum, spleen, liver, and biliary system. Such injury is always an accident and an effort is needed in preventing them. If it happens, early identification and treatment is very vital.
Colonic injury
Colonic injury happens in about 1% percutaneous renal procedures in prone position. It is thought to be due to retro-renal position of the colon. It is more common on the left side when a lower calyx access is attempted[86]. Thin patients, elderly age group, dilated colon, prior colon surgery or disease, and the presence of a horseshoe kidney are additional risk factors[40,87]. It can also happen in patients who undergo significant weight loss in a short time like patients after bariatric surgery, ileal diseases and resections. A recent hypothesis proposed retro-renal colon to be an acquired anomaly[88]. Five patients developed colonic injury in the 2nd stage PCNL. All these patients had a long-standing large hydronephrosis that was initially drained by either a nephrostomy or a DJ stent. They proposed that the colonic mesocolon lengthens over the gradually dilating obstructed kidney. Once the kidney is de-obstructed, the kidney reduces in size but the long mesocolon persists. The colon with the long mesocolon drops posterior to kidney forming a retro renal colon.
Prevention of colonic injury is very difficult. In patients who are predisposed to colonic injury, a pre-operative CT scan in prone position could help identify the position of colon in relation to the proposed tract. Awareness of the colonic gas bubble on fluoroscopy at the time of making access and monitoring any changes in the bubble could help prevent this injury. It would be possible to identify the overlying colon if a sonography guided puncture is attempted.
Liver and splenic injury
Injury to normal sized liver and spleen are very rare during PCNL and are likely to occur if the puncture is above the 10th rib[89,90]. In patients with significant splenomegaly and hepatomegaly pre-operative CT scan could be used to decide a safe access. In rare situations, CT guided access could be made. Pre-operative awareness and planning is the only way to prevent these injuries.
Pleural injury
Pleural injury is a definite risk associated with supra-costal access. All supra costal tracts traverse the diaphragm and hence there is risk of damage to the pleura and lung. The surgeon should be aware of this risk while undertaking Supracostal puncture[91]. The risk increases as the tract moves higher on the intercostal space. The risk that is about 4% in supra 12th rib access increases to nearly 20% in supra 11 rib access[92] .
To understand pleural injury during PCNL, it is important to understand the pleural anatomy. The parietal pleura crosses the 12th rib such that the medial half is covered by the pleura while the lateral half of the rib is not covered by the pleura. In the mid scapular line, while the parietal pleura is at the level of the 12th rib and the visceral pleura is at the level of the 10th rib,. The parietal and visceral pleura ascend cranially and laterally on the ribs, and further rise in deep expiration[91,93].
To prevent pleural violation[91]: (1) Make the Tract lateral to the mid scapular line; (2) As far as feasible, stay below the 10th rib; (3) Tract making should be performed in deep expiration; and (4) Tract should be kept to the minimum necessary size.
Based on the above mentioned anatomical caveats it would be rational to suggest that tracts below the 11th rib made lateral to the mid scapular line would miss not only the visceral pleura but mal also miss the parietal pleura. Tracts made through the parietal pleura may not be of clinical significance. Use of an Amplatz sheath would further mitigate major complications by preventing leakage of the irrigation fluid in the pleural space.
An anesthetist who understands the procedure and is involved during the procedure is important, as he would maintain the patient in deep expiration when the tract is being made. If the individual case demands higher tract, there is no harm in making it. Use of thoracosopy control would make this safer[94]. The pleural fluid collection, if occurs, can be easily managed by placing a chest drain at end of the procedure. It is vital to check the costo-phrenic angle at the end of the supra-costal access. A clear costo phrenic angle on fluoroscopy at the end of the procedure is a proof that pleura have not been violated[95].
PROBLEMS DURING ACCESS
Failure to opacify the system
Cause: This uncommon occurrence can occur either due to the ureteric catheter slipping out or a tightly impacted calculus preventing passage of contrast across it. Prevention: The ureteric catheter needs to be fixed to the per urethral catheter inserted initially so that it does not slip out while making the patient prone. Using a hydrophilic glide wire also helps in negotiating the wire and then the ureteric catheter across the calculus.
Remedy: For a tightly impacted calculus, where the contrast does not go across it. Keeping the patient in “head-low” position or reducing the concentration of the contrast (increase the dilution) may help some contrast go beyond the blocking calculus. If this does not work, then either an ultrasound guided puncture can be attempted or one can use a Chiba needle to opacify the system. Chiba needle is a much finer needle as compared to the initial puncture needle hence is likely to be less traumatic[96]. The needle is introduced around 2 cm lateral to the vertebral transverse process at L1-2 level. At this site it is likely to hit the renal pelvis. The CT scan images could help in identification of exact site of renal pelvis. Once the PCS is entered with a Chiba needle (confirmed by aspiration of urine from PCS), opacify the system and make the standard tract through the chosen calyx.
Extravasation of contrast
Cause: Extravasation of the contrast is an unfortunate problem. It is important to avoid this situation, as extravasation would happen before the main procedure begins, and would complicate the further access making. The extravasated contrast would make the tract making difficult and also hamper the radiologic confirmation of stone clearance post procedure. The most common cause is when an enthusiastic assistant instills a large volume of contrast under high pressure. Rarely, the contrast may extravasate from an improperly placed ureteric catheter. This would be more common in patients with large impacted ureteric calculi with infection. It may also happen intra-op when the first attempt at needle insertion is not satisfactory and the contrast leaks from the needle puncture site that is made in the collecting system.
Prevention: To prevent extravasation of contrast, inject diluted contrast slowly while keeping the ureteric catheter in the pelvis so that sudden distension of the system with consequent extravasation does not occur. It is extremely important to instruct the assistant to instill a small amount of contrast gradually at a very low pressure. The volume of the normal collecting system is 5-8 mL; hence gradual instillation of small volume is vital.
Remedy: The problem can be salvaged in multiple ways: (1) Give diuretic and wait for the contrast to get absorbed. The concentration of the extravasated contrast would significantly reduce if you wait for about 15 minutes after a frusemide injection; (2) Use concentrated contrast that would help in identification of the PCS through the dilute extravasated contrast. The tract needs to be made fast before the concentrated contrast extravasates and compounds the problem; (3) Use of air-pyelogram to identify the PCS. The similar problem of air extravasation can happen through the needle hole in the cortex; (4) Ultrasound guided percutaneous access is a good option. However, even this technique would be difficult after contrast extravasates. Do not attempt air pyelogram, if you want to do an ultrasonography; (5) Very rarely, it may be desirable to stage the procedure and re-attempt access after 48 h; (6) Grasso et al[97] initially described ureteroscopically assisted percutaneous renal access as a salvage procedure in difficult cases. This can be utilized in cases where significant extravasation has occurred. The major hindrance is the availability of a flexible scope, which is not the case in many developing countries; and (7) Giannakopoulos et al[98] have described the use of an angiographic catheter to salvage such situation. A 0.038-inch guide wire is passed through open-end ureteral catheter which is then removed and an angled- tip angiographic catheter is passed. The radiopaque tip of the angiographic catheter is easily seen on the fluoroscopy despite significant extravasation of contrast. A guide wire is then passed through the angiographic catheter. It is manipulated and brought in a calyx which is to be punctured. The angiographic catheter is then brought till the calyx and the puncture is made aiming at the tip of the catheter. The intravenous urogram film or an initial normal fluoroscopic image before extravasation, which is captured on the second monitor of the fluoroscopy unit is very helpful in manipulating the guide wire and catheter in the proper calyx. The correct position of the catheter in a posterior calyx can also be confirmed by rotating the C-arm[98].
Inability to puncture
Cause: Inability to puncture is often a technical problem .This happens either due to the inexperience of the operator or due to technical difficulty commonly while attempting puncture of a non-dilated system. This could be related to incorrect choice of the calyx for puncture.
Prevention: In the initial learning phase, presence of a more experienced colleague goes a long way in minimizing the learning curve and overcoming difficulties during the procedure. It is important to keep the PCS adequately filled for ease of puncture. Ask the assistant to continuously flush fluid in the ureteric catheter so that the system remains distended. If despite multiple attempts it is still difficult, reassess the pre-operative radiological studies and re-plan the puncture. Add a drop of methylene blue or betadine solution to the contrast; aspiration of the colored fluid (blue or brown) from kidney would give confidence of correct puncture[99].
Remedy: In a non-dilated system, fluoroscopy guided puncture is usually feasible using the techniques described above. If the surgeon is worried about trauma to the kidney, then it is prudent to use a 21 G needle for initial puncture and pass a 0.018 inch guide wire, which can be exchanged for a 0.035 inch stiffer guide wire[40]. If these attempts fail, take the help of a senior colleague from the department. An interventional radiologist may help with difficult punctures. Re-planning the procedure under CT scan guidance or ultrasonography guidance may rarely be needed[100] Puncture would also be difficult in a patient who has a very thin renal cortex. The renal cortex tends to move away from the needle or to get tented by the needle rather than getting punctured. Forcefully pushing the needle in, once near the cortex, can help the needle enter the PCS. Forceful insertion would be safe as in this patient with thin renal cortex the PCS is likely to be hugely dilated. Proper care of the wire once inserted is very important in these patients. It is also important to keep a safety wire if possible. If there were a tract loss, it would be very difficult to get inside these deflated hydronephrotic sacs. Puncture will also be awkward in very thin patients. As there is no perirenal fat pad, the kidney tends to get pushed by the needle. A bolster kept below the kidney can hold the kidney in place so that puncture can be made.
Blood at tip of needle and not urine
Cause: It is not uncommon to have made a puncture and after removing the trocar and aspiration have blood and not urine. This happens if the needle is in a blood vessel or needle is in renal parenchyma instead of in the pelvicalyceal system. It can also occur if multiple attempts have been made to achieve access.
Prevention: Avoid trauma by making multiple attempts of puncture using a 18 G needle, instead use a 21 G needle. Follow a proper puncture technique so that we aim at the calyx through the fornix.
Remedy: If after injecting saline through the ureteric catheter, the efflux clears then it suggests that the needle tip is in the collecting system and glide wire can be passed in the collecting system. If the efflux or the aspirated fluid is frank blood then the needle’s position should be readjusted. This entails with drawing the needle and adjusting the medio-lateral and anteroposterior position. It is usually the depth of the needle which needs to be adjusted. Either the needle is superficial or deep to the desired calyx. This adjustment is best made after withdrawing the needle outside the parenchyma. Manipulations within the parenchyma cause trauma and should be avoided. The 3 finger technique described by Shergill et al[46] is an attempt to help the junior trainee to overcome this difficulty. The tip of the needle should be towards the desired calyx with the C arm in the anteroposterior position or tilted towards or away from the surgeon or tilted in cephalo caudal direction. The surgeon should remember the medio-lateral adjustments should be made with the C arm at 00 and the depth adjustment should be made after tilting the C arm towards or away from the surgeon, as in Bull’s eye technique or tilting it in the cephalo-caudal direction, as in the triangulation technique. An easy way to determine the depth would be to place another needle on the skin surface over the target calyx. If the calyx is between the two needles than the puncture needle is deep and should be adjusted superficially. If the target calyx is below the two needles, then the puncture needle is superficial and should be adjusted towards the depth. Use of the Hybrid Technique described above minimizes these problems.
Inability to park the guide wire
Cause: This occurs either if the glide wire is outside the collecting system or if the calyx is completely occupied by a calculus. Rarely, inadvertent puncture of a renal cyst and aspiration of clear fluid can cause a mistaken assumption of a good puncture. But the glide wire does not enter the collecting system in such cases. If an anterior calyx s punctured then also the glide wire will not enter the pelvis easily (Figure 10B).
Prevention: Free flow of urine from the needle usually is a sure shot sign of a correct puncture. A hydrophilic glide wire usually passes easily in the pelvis even across an impacted calculus. As regards inadvertent cyst puncture, the ultrasound findings and the intravenous urogram or the CT scan picture should alert the surgeon regarding such a possibility.
Remedy: Re puncture or re-insert the glide wire if one suspects that the glide wire is outside the system. Injecting diluted contrast or diluted methylene blue can also confirm that the needle is properly positioned in the desired calyx. There may be a calculus blocking the passage of the wire down the ureter, in this situation, the second best place to park the wire would be a distant calyx. If the wire does not coil in distant calyx then keep as much length as possible of the wire in the punctured calyx[99].
Kinking of guide wire
Cause: This usually occurs due to forceful dilatation in the wrong direction and/or against resistance of the initial dilator. Once the guide rod is in place this problem cannot occur.
Prevention: The wire usually kinks at the level of the thoracolumbar fascia. Hence the fascia needs to be incised well before starting the dilatation. Use of super stiff wire is recommended due to its properties to resist kinking more than the PTFE guide wire[101]. The dilatation should be in the correct direction and with adequate force. A simple rule would be to achieve the 2/3 of the progress of the dilator by rotational screwing movements and 1/3 by force. If there is doubt regarding the correct direction then moving the glide wire gives a good indication. If the wire moves freely then it indicates that the direction and trajectory is correct. Vice versa, if the glide wire does not move freely then the direction and trajectory needs to be adjusted. This simple friction test can be of immense help in the initial learning of percutaneous renal access. Use of the 5 part PANG needle system largely avoids this problem[70].
Remedy: If a kink has occurred then the initial dilator should be advanced close to the kink and it should be pulled inside the dilator. The correct direction should then be ascertained and further dilation should be done. At times a re-puncture is needed. If a safety wire has been inserted then it can be used for dilatation. Recently Lezrek et al[102] have described a use of bi prong forceps to overcome renal mobility and prevent guide wire kinking during tract dilatation.
Under dilatation
Cause: Under dilatation is a condition when the wire was initially well placed but during dilatation the dilators and the Amplatz sheath remained short of the PCS. This usually occurs early during the learning curve. Use of Amplatz dilators is also associated with this as the terminal taper end of the dilator enters the calyx but the amplatz sheath introduced over it does not enter the calyx and remains outside the collecting system. This may cause brisk bleeding as there is a portion of parenchyma that has been partially dilated, which does not have the tamponade effects of the Amplatz sheath. This situation needs rapid management.
Prevention: Flushing saline from the ureteric catheter during the process of dilatation helps in confirming that the dilator/ dilators are within the collecting system by seeing the efflux of saline form the dilators. Small frequent bursts of screening on the C arm confirm the correct position of the dilators.
Remedy: The treatment would depend on the position of the wire. If the wire is still inside the PCS, thread the guide rod on the wire so that the bulbous end of the rod is fluoroscopically positioned in the PCS. Using the flexible guide rod may be easy in this situation. Once the guide rod is placed, use Amplatz dilators to dilate the remaining non dilated tract and the reposition the Amplatz sheath in the collecting system. If the wire has also slipped out and the nephroscope is outside the parenchyma in the perirenal fat, it is important to find the hole in the renal capsule through which partial dilatation had been done. This can be identified as a site of bleeding in the parenchyma. To identify this site one may need to reduce the irrigation pressure of the nephroscope so that venous bleeding is visible. Once the site is identified, place the guide rod through the capsular hole and confirm its position on the fluoroscopy. A guide wire may be placed through the rod to make the access secure. Once this is done, the remaining dilatation could continue with Amplatz dilators. If no bleeding site is identified, for identifying the capsular hole some colored fluid will be needed. Methylene blue or betadine solution can be used. The methylene blue solution should be very dilute. Add just 1-2 drops of methylene blue in 10 cc normal saline. For betadine solution, undiluted betadine or betadine with one-in-one dilution could be used. Flush either solution through the ureteric catheter. Watch for egress of colored solution. Once identified, place a guide rod through that site and continue with the remaining dilatation. If despite colored solution, puncture site cannot be identified, attempts re-puncture and repeat tract making. This may be difficult as the contrast may extravasate or the calyx may not fill due to leakage of contrast. Choosing an access through another calyx or sonography guided puncture may help. In a rare situation, it may be needed to stage the procedure. The puncture site seals in 48-72 h and a repeat procedure can be done after that time.
Overdilatation
Cause: Over dilatation is a state when the dilators have traversed the opposite wall of the PCS and the Amplatz sheath is now placed anterior to the kidney. Forceful dilatation is the usual reason for this problem.
Prevention: Hold the guide rod firmly and dilate 2/3 by rotation and 1/3 by force. Attempt should be to dilate till the calyx and not till the calculus. It is better to under dilate than to over dilate.
Remedy: The problem with this complication is that when the Amplatz sheath is withdrawn back to get it in the PCS, the dilated anterior wall will not have any tamponade effect and is likely to bleed briskly. Also, the irrigation fluid would leak through the hole in anterior wall. Further Nephroscopy would become difficult as the PCS may collapse due to fluid leakage. The fluid that extravasates would cause significant fluid overload. Even the stone or stone fragments can migrate outside the PCS through the hole in the anterior wall. The Amplatz sheath needs to be withdrawn gradually till the sheath is back in PCS. The further plan after this would depend on the size of perforation and the amount of bleeding. If over dilatation has resulted in a small pelvic perforation than one can get back properly in the system and quickly finish the procedure without causing much extravasation. However if there is a large perforation or significant bleeding than it is prudent to insert a nephrostomy tube, abandon the procedure and live to fight another day. Always a keep a large bore nephrostomy tube during such situations. The second procedure can be staged after 3-4 d, as the perforation usually heals within this period[103].
Loss of tract
Cause: Loss of tract means initially that the tract was well made but during Nephroscopy or lithotripsy, the Amplatz sheath has slipped out of the PCS. This happens when the glide wire slips out before the dilatation is completed. At times during the fragmentation of the calculus, the guide wire comes out and if the amplatz is not held properly then it too can come out of the collecting system.
Prevention: To prevent loss of tract, the guide wire should be adequately parked in the collecting system or passed till the bladder. Use of a safety wire is another maneuver which can prevent a complete loss of tract. Also using a super stiff guide wire instead of a slippery hydrophilic glide wire prevents complete loss of tract.
Remedy: The treatment depends on the position of the guide wire. If either the guide wire or the safety wire is well placed, it is possible to follow the wire endoscopically till the nephroscope is positioned in the PCS; the Amplatz sheath can be threaded over the nephroscope once the scope is well placed. If there is no wire, the steps are same as for under-dilatation. Look for the bleeding site or look for egress of the colored fluid. One important concern of the lost tract is that calculi or calculi fragments may extrude and can get misplaced in the perirenal fat. These calculi can be a source of persistent infection. If possible, an attempt should be made to remove all calculi. During endoscopic exploration of the perirenal space, reduce the irrigation pressure so that patient does not land in fluid overload. If despite care, the extruded calculi are misplaced in the perirenal fat, it is important to document that the radio-opaque shadows seen on post-op imaging are outside PCS. They would cause apprehension to the patient hence proper explanation to the patient is vital.
CONCLUSION
“Good results of surgery are results of good surgery”. This adage is most appropriate for a percutaneous renal access, for a correct access decides to a large extent the success of PCNL. More innovations would come in the management of renal calculus and technology would make percutaneous access easier in future; but adherence to the understanding of renal anatomy would still be the base on which a percutaneous renal access would be based.
Footnotes
P- Reviewer: Louboutin JP, Taheri S, Verma S S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
Conflict-of-interest: The authors declare that they have no competing interests.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 27, 2014
First decision: September 17, 2014
Article in press: November 27, 2014
References
- 1.Rupel E, Brown R. Nephroscopy with removal of stone following nephrostomy for obstructing calculus anuria. J Urol. 1941;46:177–182. [Google Scholar]
- 2.Goodwin WE, Casey WC, Woolf W. Percutaneous trocar (needle) nephrostomy in hydronephrosis. J Am Med Assoc. 1955;157:891–894. doi: 10.1001/jama.1955.02950280015005. [DOI] [PubMed] [Google Scholar]
- 3.Bissada NK, Meacham KR, Redman JF. Nephrostoscopy with removal of renal pelvic calculi. J Urol. 1974;112:414–416. doi: 10.1016/s0022-5347(17)59747-5. [DOI] [PubMed] [Google Scholar]
- 4.Karametchi A, O’donnell WF. Percutaneous nephrolithotomy: an innovative extraction technique. J Urol. 1977;118:671. doi: 10.1016/s0022-5347(17)58148-3. [DOI] [PubMed] [Google Scholar]
- 5.Fernström I, Johansson B. Percutaneous pyelolithotomy. A new extraction technique. Scand J Urol Nephrol. 1976;10:257–259. doi: 10.1080/21681805.1976.11882084. [DOI] [PubMed] [Google Scholar]
- 6.Morris DS, Wei JT, Taub DA, Dunn RL, Wolf JS, Hollenbeck BK. Temporal trends in the use of percutaneous nephrolithotomy. J Urol. 2006;175:1731–1376. doi: 10.1016/S0022-5347(05)00994-8. [DOI] [PubMed] [Google Scholar]
- 7.de la Rosette J, Assimos D, Desai M, Gutierrez J, Lingeman J, Scarpa R, Tefekli A. The Clinical Research Office of the Endourological Society Percutaneous Nephrolithotomy Global Study: indications, complications, and outcomes in 5803 patients. J Endourol. 2011;25:11–17. doi: 10.1089/end.2010.0424. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal M, Agrawal MS, Jaiswal A, Kumar D, Yadav H, Lavania P. Safety and efficacy of ultrasonography as an adjunct to fluoroscopy for renal access in percutaneous nephrolithotomy (PCNL) BJU Int. 2011;108:1346–1349. doi: 10.1111/j.1464-410X.2010.10002.x. [DOI] [PubMed] [Google Scholar]
- 9.Ray AA, Chung DG, Honey RJ. Percutaneous nephrolithotomy in the prone and prone-flexed positions: anatomic considerations. J Endourol. 2009;23:1607–1614. doi: 10.1089/end.2009.0294. [DOI] [PubMed] [Google Scholar]
- 10.Tepeler A, Armağan A, Akman T, Polat EC, Ersöz C, Topaktaş R, Erdem MR, Onol SY. Impact of percutaneous renal access technique on outcomes of percutaneous nephrolithotomy. J Endourol. 2012;26:828–833. doi: 10.1089/end.2011.0563. [DOI] [PubMed] [Google Scholar]
- 11.Duty B, Waingankar N, Okhunov Z, Ben Levi E, Smith A, Okeke Z. Anatomical variation between the prone, supine, and supine oblique positions on computed tomography: implications for percutaneous nephrolithotomy access. Urology. 2012;79:67–71. doi: 10.1016/j.urology.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 12.Lashley DB, Fuchs EF. Urologist-acquired renal access for percutaneous renal surgery. Urology. 1998;51:927–931. doi: 10.1016/s0090-4295(98)00101-0. [DOI] [PubMed] [Google Scholar]
- 13.Tomaszewski JJ, Ortiz TD, Gayed BA, Smaldone MC, Jackman SV, Averch TD. Renal access by urologist or radiologist during percutaneous nephrolithotomy. J Endourol. 2010;24:1733–1737. doi: 10.1089/end.2010.0191. [DOI] [PubMed] [Google Scholar]
- 14.El-Assmy AM, Shokeir AA, Mohsen T, El-Tabey N, El-Nahas AR, Shoma AM, Eraky I, El-Kenawy MR, El-Kappany HA. Renal access by urologist or radiologist for percutaneous nephrolithotomy--is it still an issue? J Urol. 2007;178:916–920; discussion 920. doi: 10.1016/j.juro.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Bird VG, Fallon B, Winfield HN. Practice patterns in the treatment of large renal stones. J Endourol. 2003;17:355–363. doi: 10.1089/089277903767923119. [DOI] [PubMed] [Google Scholar]
- 16.Lee CL, Anderson JK, Monga M. Residency training in percutaneous renal access: does it affect urological practice? J Urol. 2004;171:592–595. doi: 10.1097/01.ju.0000104849.25168.6d. [DOI] [PubMed] [Google Scholar]
- 17.Miller J, Durack JC, Sorensen MD, Wang JH, Stoller ML. Renal calyceal anatomy characterization with 3-dimensional in vivo computerized tomography imaging. J Urol. 2013;189:562–567. doi: 10.1016/j.juro.2012.09.040. [DOI] [PubMed] [Google Scholar]
- 18.Edgcombe H, Carter K, Yarrow S. Anaesthesia in the prone position. Br J Anaesth. 2008;100:165–183. doi: 10.1093/bja/aem380. [DOI] [PubMed] [Google Scholar]
- 19.Stevens WR, Glazer PA, Kelley SD, Lietman TM, Bradford DS. Ophthalmic complications after spinal surgery. Spine (Phila Pa 1976) 1997;22:1319–1324. doi: 10.1097/00007632-199706150-00008. [DOI] [PubMed] [Google Scholar]
- 20.Miller NL, Matlaga BR, Lingeman JE. Techniques for fluoroscopic percutaneous renal access. J Urol. 2007;178:15–23. doi: 10.1016/j.juro.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Ko R, Soucy F, Denstedt JD, Razvi H. Percutaneous nephrolithotomy made easier: a practical guide, tips and tricks. BJU Int. 2008;101:535–539. doi: 10.1111/j.1464-410X.2007.07259.x. [DOI] [PubMed] [Google Scholar]
- 22.Sampaio FJ. Renal anatomy. Endourologic considerations. Urol Clin North Am. 2000;27:585–607. doi: 10.1016/s0094-0143(05)70109-9. [DOI] [PubMed] [Google Scholar]
- 23.Carrico CW, Zerin JM. Sonographic measurement of renal length in children: does the position of the patient matter? Pediatr Radiol. 1996;26:553–555. doi: 10.1007/BF01372240. [DOI] [PubMed] [Google Scholar]
- 24.Sharma G, Sharma A, Maheshwari P. Predictive value of decreased renal pelvis anteroposterior diameter in prone position for prenatally detected hydronephrosis. J Urol. 2012;187:1839–1843. doi: 10.1016/j.juro.2011.12.093. [DOI] [PubMed] [Google Scholar]
- 25.Park S, Pearle MS. Imaging for percutaneous renal access and management of renal calculi. Urol Clin North Am. 2006;33:353–364. doi: 10.1016/j.ucl.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Thiruchelvam N, Mostafid H, Ubhayakar G. Planning percutaneous nephrolithotomy using multidetector computed tomography urography, multiplanar reconstruction and three-dimensional reformatting. BJU Int. 2005;95:1280–1284. doi: 10.1111/j.1464-410X.2005.05519.x. [DOI] [PubMed] [Google Scholar]
- 27.Hopper KD, Yakes WF. The posterior intercostal approach for percutaneous renal procedures: risk of puncturing the lung, spleen, and liver as determined by CT. AJR Am J Roentgenol. 1990;154:115–117. doi: 10.2214/ajr.154.1.2104692. [DOI] [PubMed] [Google Scholar]
- 28.Ghani KR, Patel U, Anson K. Computed tomography for percutaneous renal access. J Endourol. 2009;23:1633–1639. doi: 10.1089/end.2009.1529. [DOI] [PubMed] [Google Scholar]
- 29.Bilen CY, Koçak B, Kitirci G, Danaci M, Sarikaya S. Simple trigonometry on computed tomography helps in planning renal access. Urology. 2007;70:242–245; discussion 245. doi: 10.1016/j.urology.2007.03.079. [DOI] [PubMed] [Google Scholar]
- 30.Sampaio FJ, Zanier JF, Aragão AH, Favorito LA. Intrarenal access: 3-dimensional anatomical study. J Urol. 1992;148:1769–1773. doi: 10.1016/s0022-5347(17)37024-6. [DOI] [PubMed] [Google Scholar]
- 31.Schultheiss D, Engel RM, Crosby RW, Lees GP, Truss MC, Jonas U. Max Brödel (1870-1941) and medical illustration in urology. J Urol. 2000;164:1137–1142. [PubMed] [Google Scholar]
- 32.Settlage J. Derek Hodson: Teaching and Learning About Science: Language, Theories, Methods, History, Traditions and Value. Science & Education. 2011;20:393–396. [Google Scholar]
- 33.Kaye KW, Reinke DB. Detailed caliceal anatomy for endourology. J Urol. 1984;132:1085–1088. doi: 10.1016/s0022-5347(17)50042-7. [DOI] [PubMed] [Google Scholar]
- 34.Sampaio FJ, Mandarim-De-Lacerda CA, De Aragão AH. [The collector system of the kidney. Applied anatomy based on the analysis of 3-dimensional casts] J Urol (Paris) 1987;93:183–185. [PubMed] [Google Scholar]
- 35.Sampaio FJ. Renal collecting system anatomy: its possible role in the effectiveness of renal stone treatment. Curr Opin Urol. 2001;11:359–366. doi: 10.1097/00042307-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Hwang TK. Percutaneous nephroscopic surgery. Korean J Urol. 2010;51:298–307. doi: 10.4111/kju.2010.51.5.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eisner BH, Cloyd J, Stoller ML. Lower-pole fluoroscopy-guided percutaneous renal access: which calix is posterior? J Endourol. 2009;23:1621–1625. doi: 10.1089/end.2009.1527. [DOI] [PubMed] [Google Scholar]
- 38.Osman M, Wendt-Nordahl G, Heger K, Michel MS, Alken P, Knoll T. Percutaneous nephrolithotomy with ultrasonography-guided renal access: experience from over 300 cases. BJU Int. 2005;96:875–878. doi: 10.1111/j.1464-410X.2005.05749.x. [DOI] [PubMed] [Google Scholar]
- 39.Abolhassani N, Patel R, Moallem M. Needle insertion into soft tissue: a survey. Med Eng Phys. 2007;29:413–431. doi: 10.1016/j.medengphy.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Wolf JS. Percutaneous approaches to the upper urinary tract collecting system. Campbell-Walsh Urology. 10th ed. Philadelphia, PA: Saunders Elsevier; 2011. [Google Scholar]
- 41.Bernardo NO. Percutaneous Renal Access Under Fluoroscopic Control. Smith’s Textbook of Endourology. 3rd ed. Available from: http://onlinelibrary.wiley.com/book/10.1002/9781444345148.
- 42.Steinberg PL, Semins MJ, Wason SE, Matlaga BR, Pais VM. Fluoroscopy-guided percutaneous renal access. J Endourol. 2009;23:1627–1631. doi: 10.1089/end.2009.1528. [DOI] [PubMed] [Google Scholar]
- 43.Bilen CY, Aşçi R, Sarikaya S, Büyükalpelli R, Yilmaz AF. Laser-assisted fluoroscopic puncture: a new technique for accessing the kidney. J Endourol. 2003;17:485–491. doi: 10.1089/089277903769013630. [DOI] [PubMed] [Google Scholar]
- 44.Ko R, Razvi H. C-arm laser positioning device to facilitate percutaneous renal access. Urology. 2007;70:360–361. doi: 10.1016/j.urology.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Tanriverdi O, Boylu U, Kendirci M, Kadihasanoglu M, Horasanli K, Miroglu C. The learning curve in the training of percutaneous nephrolithotomy. Eur Urol. 2007;52:206–211. doi: 10.1016/j.eururo.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Shergill IS, Abdulmajed MI, Moussa SA, Rix GH. The 3-finger technique in establishing percutaneous renal access: a new and simple method for junior trainees. J Surg Educ. 2012;69:550–553. doi: 10.1016/j.jsurg.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Mues E, Gutiérrez J, Loske AM. Percutaneous renal access: a simplified approach. J Endourol. 2007;21:1271–1275. doi: 10.1089/end.2007.9887. [DOI] [PubMed] [Google Scholar]
- 48.Liatsikos EN, Kapoor R, Lee B, Jabbour M, Barbalias G, Smith AD. “Angular percutaneous renal access”. Multiple tracts through a single incision for staghorn calculous treatment in a single session. Eur Urol. 2005;48:832–837. doi: 10.1016/j.eururo.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 49.Mozer P, Conort P, Leroy A, Baumann M, Payan Y, Troccaz J, Chartier-Kastler E, Richard F. Aid to percutaneous renal access by virtual projection of the ultrasound puncture tract onto fluoroscopic images. J Endourol. 2007;21:460–465. doi: 10.1089/end.2006.0168. [DOI] [PubMed] [Google Scholar]
- 50.Soria F, Delgado MI, Sánchez FM, Allona A, Jiménez Cruz JF, Morell E, Usón J. Effectiveness of three-dimensional fluoroscopy in percutaneous nephrostomy: an animal model study. Urology. 2009;73:649–652; discussion 652-654. doi: 10.1016/j.urology.2008.09.063. [DOI] [PubMed] [Google Scholar]
- 51.Cadeddu JA, Bzostek A, Schreiner S, Barnes AC, Roberts WW, Anderson JH, Taylor RH, Kavoussi LR. A robotic system for percutaneous renal access. J Urol. 1997;158:1589–1593. [PubMed] [Google Scholar]
- 52.Bauer J, Lee BR, Stoianovici D, Bishoff JT, Micali S, Micali F, Kavoussi LR. Remote percutaneous renal access using a new automated telesurgical robotic system. Telemed J E Health. 2001;7:341–346. doi: 10.1089/15305620152814746. [DOI] [PubMed] [Google Scholar]
- 53.Su LM, Stoianovici D, Jarrett TW, Patriciu A, Roberts WW, Cadeddu JA, Ramakumar S, Solomon SB, Kavoussi LR. Robotic percutaneous access to the kidney: comparison with standard manual access. J Endourol. 2002;16:471–475. doi: 10.1089/089277902760367421. [DOI] [PubMed] [Google Scholar]
- 54.Li X, Liao S, Yu Y, Dai Q, Song B, Li L. Stereotactic localisation system: a modified puncture technique for percutaneous nephrolithotomy. Urol Res. 2012;40:395–401. doi: 10.1007/s00240-011-0434-2. [DOI] [PubMed] [Google Scholar]
- 55.Hatipoglu NK, Bodakci MN, Penbegül N, Bozkurt Y, Sancaktutar AA, Atar M, Söylemez H. Monoplanar access technique for percutaneous nephrolithotomy. Urolithiasis. 2013;41:257–263. doi: 10.1007/s00240-013-0557-8. [DOI] [PubMed] [Google Scholar]
- 56.Lang EK. Percutaneous nephrostolithotomy and lithotripsy: a multi-institutional survey of complications. Radiology. 1987;162:25–30. doi: 10.1148/radiology.162.1.3786771. [DOI] [PubMed] [Google Scholar]
- 57.Sharma G, Sharma A. Determining site of skin puncture for percutaneous renal access using fluoroscopy-guided triangulation technique. J Endourol. 2009;23:193–195. doi: 10.1089/end.2008.0170. [DOI] [PubMed] [Google Scholar]
- 58.Handa RK, Matlaga BR, Connors BA, Ying J, Paterson RF, Kuo RL, Kim SC, Lingeman JE, Evan AP, Willis LR. Acute effects of percutaneous tract dilation on renal function and structure. J Endourol. 2006;20:1030–1040. doi: 10.1089/end.2006.20.1030. [DOI] [PubMed] [Google Scholar]
- 59.Davidoff R, Bellman GC. Influence of technique of percutaneous tract creation on incidence of renal hemorrhage. J Urol. 1997;157:1229–1231. [PubMed] [Google Scholar]
- 60.Ozok HU, Sagnak L, Senturk AB, Karakoyunlu N, Topaloglu H, Ersoy H. A comparison of metal telescopic dilators and Amplatz dilators for nephrostomy tract dilation in percutaneous nephrolithotomy. J Endourol. 2012;26:630–634. doi: 10.1089/end.2011.0291. [DOI] [PubMed] [Google Scholar]
- 61.Falahatkar S, Neiroomand H, Akbarpour M, Emadi SA, Khaki N. One-shot versus metal telescopic dilation technique for tract creation in percutaneous nephrolithotomy: comparison of safety and efficacy. J Endourol. 2009;23:615–618. doi: 10.1089/end.2008.0330. [DOI] [PubMed] [Google Scholar]
- 62.Li Y, Yang L, Xu P, Shen P, Qian S, Wei W, Wang J. One-shot versus gradual dilation technique for tract creation in percutaneous nephrolithotomy: A systematic review and meta-analysis. Urol Res. 2013;41:443–448. doi: 10.1007/s00240-013-0583-6. [DOI] [PubMed] [Google Scholar]
- 63.Dehong C, Liangren L, Huawei L, Qiang W. A comparison among four tract dilation methods of percutaneous nephrolithotomy: A systematic review and meta-analysis. Urolithiasis. 2013;41:523–530. doi: 10.1007/s00240-013-0598-z. [DOI] [PubMed] [Google Scholar]
- 64.Wezel F, Mamoulakis C, Rioja J, Michel MS, de la Rosette J, Alken P. Two contemporary series of percutaneous tract dilation for percutaneous nephrolithotomy. J Endourol. 2009;23:1655–1661. doi: 10.1089/end.2009.0213. [DOI] [PubMed] [Google Scholar]
- 65.Gönen M, Istanbulluoglu OM, Cicek T, Ozturk B, Ozkardes H. Balloon dilatation versus Amplatz dilatation for nephrostomy tract dilatation. J Endourol. 2008;22:901–904. doi: 10.1089/end.2007.0167. [DOI] [PubMed] [Google Scholar]
- 66.Safak M, Gokus C, Soygur T. Nephrostomy tract dilation using a balloon dilator in percutaneous renal surgery: Experience with 95 cases and comparison with the fascial dilator system. Urol Int. 2003;71:382–384. doi: 10.1159/000074090. [DOI] [PubMed] [Google Scholar]
- 67.Al-Kandari AM, Jabbour M, Anderson A, Shokeir AA, Smith AD. Comparative study of degree of renal trauma between Amplatz sequential fascial dilation and balloon dilation during percutaneous renal surgery in an animal model. Urology. 2007;69:586–589. doi: 10.1016/j.urology.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 68.Chiang PH, Su HH. Randomized and prospective trial comparing tract creation using plasma vaporization with balloon dilatation in percutaneous nephrolithotomy. BJU Int. 2013;112:89–93. doi: 10.1111/j.1464-410X.2012.11507.x. [DOI] [PubMed] [Google Scholar]
- 69.Goharderakhshan RZ, Schwartz BF, Rudnick DM, Irby PB, Stoller ML. Radially expanding single-step nephrostomy tract dilator. Urology. 2001;58:693–696. doi: 10.1016/s0090-4295(01)01359-0. [DOI] [PubMed] [Google Scholar]
- 70.Patil AV. A novel 5-part Percutaneous Access Needle with Glidewire technique (5-PANG) for percutaneous nephrolithotomy: our initial experience. Urology. 2010;75:1206–1208. doi: 10.1016/j.urology.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 71.Abraham JB, Gamboa AJ, Finley DS, Beck SM, Lee HJ, Santos RJ, Box GN, Deane LA, Vajgrt DJ, McDougall EM, et al. he UCI Seldinger technique for percutaneous renal cryoablation: protecting the tract and achieving hemostasis. J Endourol. 2009;23:43–49. doi: 10.1089/end.2008.0032. [DOI] [PubMed] [Google Scholar]
- 72.Muslumanoglu AY, Tefekli A, Karadag MA, Tok A, Sari E, Berberoglu Y. Impact of percutaneous access point number and location on complication and success rates in percutaneous nephrolithotomy. Urol Int. 2006;77:340–346. doi: 10.1159/000096339. [DOI] [PubMed] [Google Scholar]
- 73.Williams SK, Leveillee RJ. Management of staghorn calculus: single puncture with judicious use of the flexible nephroscope. Curr Opin Urol. 2008;18:224–228. doi: 10.1097/MOU.0b013e3282f517c0. [DOI] [PubMed] [Google Scholar]
- 74.Golijanin D, Katz R, Verstandig A, Sasson T, Landau EH, Meretyk S. The supracostal percutaneous nephrostomy for treatment of staghorn and complex kidney stones. J Endourol. 1998;12:403–405. doi: 10.1089/end.1998.12.403. [DOI] [PubMed] [Google Scholar]
- 75.Aron M, Yadav R, Goel R, Kolla SB, Gautam G, Hemal AK, Gupta NP. Multi-tract percutaneous nephrolithotomy for large complete staghorn calculi. Urol Int. 2005;75:327–332. doi: 10.1159/000089168. [DOI] [PubMed] [Google Scholar]
- 76.Ferrandino MN, Bagrodia A, Pierre SA, Scales CD, Rampersaud E, Pearle MS, Preminger GM. Radiation exposure in the acute and short-term management of urolithiasis at 2 academic centers. J Urol. 2009;181:668–672; discussion 673. doi: 10.1016/j.juro.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 77.Wrixon AD. New recommendations from the International Commission on Radiological Protection--a review. Phys Med Biol. 2008;53:R41–R60. doi: 10.1088/0031-9155/53/8/R01. [DOI] [PubMed] [Google Scholar]
- 78.Bowsher WG, Blott P, Whitfield HN. Radiation protection in percutaneous renal surgery. Br J Urol. 1992;69:231–233. doi: 10.1111/j.1464-410x.1992.tb15518.x. [DOI] [PubMed] [Google Scholar]
- 79.Mancini JG, Raymundo EM, Lipkin M, Zilberman D, Yong D, Bañez LL, Miller MJ, Preminger GM, Ferrandino MN. Factors affecting patient radiation exposure during percutaneous nephrolithotomy. J Urol. 2010;184:2373–2377. doi: 10.1016/j.juro.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 80.Tepeler A, Binbay M, Yuruk E, Sari E, Kaba M, Muslumanoglu AY, Tefekli A. Factors affecting the fluoroscopic screening time during percutaneous nephrolithotomy. J Endourol. 2009;23:1825–1829. doi: 10.1089/end.2009.0256. [DOI] [PubMed] [Google Scholar]
- 81.Dias J, Unsworth DJ, Walker-Smith JA. Antigliadin and antireticulin antibodies in screening for coeliac disease. Lancet. 1987;2:157–158. doi: 10.1016/s0140-6736(87)92356-7. [DOI] [PubMed] [Google Scholar]
- 82.Yang RM, Morgan T, Bellman GC. Radiation protection during percutaneous nephrolithotomy: a new urologic surgery radiation shield. J Endourol. 2002;16:727–731. doi: 10.1089/08927790260472872. [DOI] [PubMed] [Google Scholar]
- 83.Elkoushy MA, Shahrour W, Andonian S. Pulsed fluoroscopy in ureteroscopy and percutaneous nephrolithotomy. Urology. 2012;79:1230–1235. doi: 10.1016/j.urology.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 84.Lipkin ME, Mancini JG, Zilberman DE, Raymundo ME, Yong D, Ferrandino MN, Miller MJ, Yoshizumi TT, Preminger GM. Reduced radiation exposure with the use of an air retrograde pyelogram during fluoroscopic access for percutaneous nephrolithotomy. J Endourol. 2011;25:563–567. doi: 10.1089/end.2010.0431. [DOI] [PubMed] [Google Scholar]
- 85.Zeng G, Zhao Z, Zhong W, Wu K, Chen W, Wu W, Xiao C, Liu Y. Evaluation of a novel fascial dilator modified with scale marker in percutaneous nephrolithotomy for reducing the X-ray exposure: a randomized clinical study. J Endourol. 2013;27:1335–1340. doi: 10.1089/end.2012.0671. [DOI] [PubMed] [Google Scholar]
- 86.El-Nahas AR, Shokeir AA, El-Assmy AM, Shoma AM, Eraky I, El-Kenawy MR, El-Kappany HA. Colonic perforation during percutaneous nephrolithotomy: study of risk factors. Urology. 2006;67:937–941. doi: 10.1016/j.urology.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 87.Korkes F, Lopes Neto AC, Lucio J, Bezerra CA, Wroklawski ER. Management of colon injury after percutaneous renal surgery. J Endourol. 2009;23:569–573. doi: 10.1089/end.2008.0506. [DOI] [PubMed] [Google Scholar]
- 88.Maheshwari PN. Retro-renal colon: Is it an acquired anomaly? J Endourol. 2011;28:A16U. [Google Scholar]
- 89.El-Nahas AR, Mansour AM, Ellaithy R, Abol-Enein H. Case report: conservative treatment of liver injury during percutaneous nephrolithotomy. J Endourol. 2008;22:1649–1652. doi: 10.1089/end.2008.0147. [DOI] [PubMed] [Google Scholar]
- 90.Shah HN, Hegde SS, Mahajan AP, Sodha H, Shah R, Bansal M. Splenic injury: rare complication of percutaneous nephrolithotomy: report of two cases with review of literature. J Endourol. 2007;21:919–922. doi: 10.1089/end.2006.0451. [DOI] [PubMed] [Google Scholar]
- 91.Maheshwari PN, Mane DA, Pathak AB. Management of pleural injury after percutaneous renal surgery. J Endourol. 2009;23:1769–1772. doi: 10.1089/end.2009.1549. [DOI] [PubMed] [Google Scholar]
- 92.Sukumar S, Nair B, Ginil KP, Sanjeevan KV, Sanjay BH. Supracostal access for percutaneous nephrolithotomy: less morbid, more effective. Int Urol Nephrol. 2008;40:263–267. doi: 10.1007/s11255-007-9270-2. [DOI] [PubMed] [Google Scholar]
- 93.Stening SG, Bourne S. Supracostal percutaneous nephrolithotomy for upper pole caliceal calculi. J Endourol. 1998;12:359–362. doi: 10.1089/end.1998.12.359. [DOI] [PubMed] [Google Scholar]
- 94.Finelli A, Honey RJ. Thoracoscopy-assisted high intercostal percutaneous renal access. J Endourol. 2001;15:581–584; discussion 584-585. doi: 10.1089/089277901750426337. [DOI] [PubMed] [Google Scholar]
- 95.Ogan K, Corwin TS, Smith T, Watumull LM, Mullican MA, Cadeddu JA, Pearle MS. Sensitivity of chest fluoroscopy compared with chest CT and chest radiography for diagnosing hydropneumothorax in association with percutaneous nephrostolithotomy. Urology. 2003;62:988–992. doi: 10.1016/j.urology.2003.07.024. [DOI] [PubMed] [Google Scholar]
- 96.Zagoria RJ, Dyer RB. Do’s and don’t’s of percutaneous nephrostomy. Acad Radiol. 1999;6:370–377. doi: 10.1016/s1076-6332(99)80233-5. [DOI] [PubMed] [Google Scholar]
- 97.Grasso M, Lang G, Taylor FC. Flexible ureteroscopically assisted percutaneous renal access. Tech Urol. 1995;1:39–43. [PubMed] [Google Scholar]
- 98.Giannakopoulos S, Bantis A, Kalaitzis C, Touloupidis S. Use of an angiographic catheter to facilitate fluoroscopy-guided percutaneous renal access in cases with diffuse contrast extravasation. J Endourol. 2010;24:1575–1578. doi: 10.1089/end.2010.0019. [DOI] [PubMed] [Google Scholar]
- 99.Rais-Bahrami S, Friedlander JI, Duty BD, Okeke Z, Smith AD. Difficulties with access in percutaneous renal surgery. Ther Adv Urol. 2011;3:59–68. doi: 10.1177/1756287211400661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lojanapiwat B. The ideal puncture approach for PCNL: Fluoroscopy, ultrasound or endoscopy? Indian J Urol. 2013;29:208–213. doi: 10.4103/0970-1591.117284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Skolarikos A, Alivizatos G, de la Rosette JJ. Percutaneous nephrolithotomy and its legacy. Eur Urol. 2005;47:22–28. doi: 10.1016/j.eururo.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 102.Lezrek M, Bazine K, Asseban M, Ammani A, Moufid K, Beddouch A, Alami M. Technique to overcome renal mobility during percutaneous tract dilatation: bi-prong forceps renal parenchyma dissection. BJU Int. 2013;112:697–702. doi: 10.1111/bju.12104. [DOI] [PubMed] [Google Scholar]
- 103.Maheshwari PN, Andankar MG, Bansal M. Nephrostomy tube after percutaneous nephrolithotomy: large-bore or pigtail catheter? J Endourol. 2000;14:735–737; discussion 737-738. doi: 10.1089/end.2000.14.735. [DOI] [PubMed] [Google Scholar]


