Abstract
BACKGROUND
Patients with structural heart disease are prone to ventricular tachycardia (VT) and ventricular fibrillation (VF), which account for the majority of sudden cardiac deaths (SCDs). We sought to examine echocardiographic parameters that can predict VT as documented by implantable cardioverter-defibrillator (ICD) appropriate discharge. We examine echocardiographic parameters other than ejection fraction that may predict VT as recorded via rates of ICD discharge.
METHODS
Analysis of 586 patients (469 males; mean age = 68 ± 3 years; mean follow-up time of 11 ± 14 months) was undertaken. Echo parameters assessed included left ventricular (LV) internal end diastolic/systolic dimension (LVIDd, LVIDs), relative wall thickness (RWT), and left atrial (LA) size.
RESULTS
The incidence of VT was 0.22 (114 VT episodes per 528 person-years of follow-up time). Median time-to-first VT was 3.8 years. VT was documented in 79 patients (59 first VT incidence, 20 multiple). The echocardiographic parameter associated with first VT was LVIDs >4 cm (P = 0.02).
CONCLUSION
The main echocardiographic predictor associated with the first occurrence of VT was LVIDs >4 cm. Patients with an LVIDs >4 cm were 2.5 times more likely to have an episode of VT. Changes in these echocardiographic parameters may warrant aggressive pharmacologic therapy and implantation of an ICD.
Keywords: ventricular tachycardia, echocardiography, implantable cardioverter-defibrillator (ICD), sudden cardiac death
Introduction
Since the introduction of the implantable cardioverter- defibrillator (ICD) approximately 25 years ago, the list of indications for implantation has progressively expanded to include a larger cohort of patients with a variety of cardiac conditions. The most commonly used quantifiable parameter for determination of candidates for ICD implantation is left ventricular ejection fraction (LVEF). While the implantation of ICDs in patients who have survived a life-threatening ventricular arrhythmia as secondary prevention of sudden cardiac death (SCD) has long been accepted, studies such as the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) and the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II) have demonstrated the clinical utility of prophylactic ICD implantation in patients with a reduced LVEF as primary prevention of SCD as well.1,2 ICD therapy is also employed in patients with ischemic cardiomyopathy for prevention of SCD, such as in patients with prior myocardial infarction (MI) with residual scar tissue, which serves as a substrate for arrhythmogenicity and potentially malignant ventricular arrhythmias.
There are numerous accepted indications for ICD implantation based upon large-scale randomized controlled trials comparing the efficacy of ICD implantation with medical therapy using antiarrhythmic agents. For patients who have experienced clinically significant ventricular arrhythmias, ICD implantation is recognized as the treatment of choice for secondary prevention of arrhythmia recurrence. Large-scale studies such as the Antiarrhythmics vs Implantable Defibrillators (AVID) trial3 and the Cardiac Arrest Study Hamburg (CASH)4 have demonstrated the relative superiority of ICDs in reducing mortality compared to antiarrhythmic pharmacotherapy. The patients with the greatest survival benefit from ICD therapy in these trials were those with diagnosed heart failure and significantly diminished LVEF.5
While most studies have demonstrated relatively good outcomes by using the LVEF as the primary indicator of benefit from ICD implantation, some studies have raised the concern that LVEF alone is not a sufficient predictor of survival benefit post-ICD implantation, particularly in certain subsets of patients, such as post-MI patients with reduced LVEF. The basis of such arguments often relies upon the multifactorial nature of ventricular arrhythmias, primarily ventricular tachycardia (VT) and ventricular fibrillation (VF). For example, in patients with significant coronary artery disease but no previous history of MI, the majority of incidents of SCD are attributed to ventricular arrhythmias arising in the setting of acute ischemia with a preserved LVEF.6,7 Using LVEF as the sole determinant of which members of this patient population would benefit from ICD therapy would exclude the majority of these patients.
There are numerous indications for ICD implantation other than reduced ejection fraction. Among the plethora of conditions for which ICD implantation might be useful are cardiac ion channel abnormalities, documented arrhythmogenicity secondary to structural heart disease, and rarer conditions such as the Brugada syndrome,8 long-QT syndrome,9 and hypertrophic cardiomyopathy.10 Despite the expanding list of indications for ICD therapy, there are few readily quantifiable parameters that can be used to definitively state that certain patients would benefit from implantation of a defibrillator. Echocardiography, which is often the initial imaging modality used to assess myocardial function, can provide a wealth of information regarding overall cardiac health and may be used to classify potential candidates for ICD implantation. The utilization of echocardiographic parameters such as left ventricular (LV) systolic/diastolic dimensions, left atrial (LA) size, and other readily quantifiable variables, when used in conjunction with LVEF, may help in both risk stratification for determining which patients could benefit most from ICD implantation and in modifying therapy, whether that involves altering ICD settings or adding or modifying antiarrhythmic pharmacotherapy.
Methods
Inclusion/exclusion criteria
Analysis of data from a total of 586 patients with a history of ICD implantation was performed. The study was approved by the institutional review board of North Shore-LIJ Health System, and conducted in accordance with the principles of the Declaration of Helsinki. The list of potential subjects was compiled from a database query of the Heath Information Management Systems database at North Shore University Hospital in Manhasset, New York. All patients greater than 18 years of age who had undergone ICD implantation at North Shore University Hospital between January 1999 and August 2005 were included regardless of the indication for ICD implantation. Only patients who followed up in the office were included in the study, so no patients were lost to follow-up. In 77 subjects (13%), the indication for implantation was for primary prevention. The remainder of the patients were implanted for secondary prevention such as LVEF less than 35%, hypertrophic obstructive cardiomyopathy, long-QT syndromes, and others, which account for SCD. Subjects in the primary prevention group were significantly older than those in the secondary prevention group (71 ± 12 vs 68 ± 12; P = 0.02). The mean age at ICD implantation for the entire population was 68 (±13 years) with an average follow-up of 11.0 ± 13.8 months.
Echocardiographic predictors were obtained from an echocardiogram performed within one month prior to the implant and were analyzed to determine potential association with incidence of VT as documented by ICD discharge in the post-implantation follow-up period. Echocardiographic parameters assessed included LV internal end diastolic and systolic dimension (LVIDd, LVIDs), relative wall thickness [RWT = (2 × posterior wall thickness)/LVIDd], and LA size to determine possible associations with the incidence of first episodes of VT. Patients had standard transthoracic echocardiography (TTE) performed using the Acuson Sequoia™ C256 (Siemens Medical, Mountain View, CA, USA).
Follow-up and endpoint variables
The starting point of the study was the time of ICD implantation. All patients were seen routinely every three months or after each device delivered electrical therapy. ICD interrogation reports were the primary means of follow-up after device implantation. Not all patients kept their clinic visit, and some patients presented before scheduled appointments because of ICD discharge or battery replacement. At each follow-up visit, the ICD provided an interrogation report and quantified each episode of arrhythmia. The patient charts were reviewed, and the electronic database within the health information management system was utilized to collect the dates of the arrhythmias and the specific type of arrhythmia recorded. The primary endpoint variable for this study was the time-to-first VT. Each episode of VT was analyzed via the stored ICD electrogram. Subjects who did not experience an episode of VT were considered censored as of their last follow-up.
Measurements of echocardiographic parameters
Specific echocardiographic parameters were assessed for purposes of data collection and analysis. All echocardiographic measurements were obtained by level III trained echocardiographers according to standard American Society of Echocardiography (ASE) recommended techniques of chamber and function quantification based upon the ASE guideline statement.11
Statistical methods for analysis of time-to-first VT
All echocardiographic parameters were considered for analysis of time-to-first VT. Comparisons of Kaplan–Meier curves were carried out using the log-rank test. A multivariate analysis, based on the Cox proportional hazards regression, was carried out, including all the variables that were found to be individually significant. A P-value <0.05 was considered to be statistically significant. All statistical tests were two sided. A backward elimination selection algorithm was subsequently used to determine which factors were most significantly associated with time-to-first VT. Baseline patient characteristics are expressed as means ± standard deviation.
Results
Baseline characteristics and indications for implantation
Baseline patient characteristics are listed in Table 1. Of the 586 patients reviewed, the indications for ICD implantation were distributed as follows: non-sustained ventricular tachycardia (NSVT) (n = 464; 80%), congestive heart failure (defined by the absence of NSVT and an ejection fraction of <30%) (n = 81; 13.9%), VF arrest (n = 39; 6.72%), and MADIT 2 criteria (n = 11; 1.9%). The incidence of VT in the entire population was 0.22 (114 VT episodes in 528 person-years). The median time-to-first episode of VT was 3.8 years. VT was documented in a total of 79 patients (59 first VT incidence, 20 recurrent VT). The only echocardiographic parameter (Table 2) associated with time-to-first VT was the LV internal systolic dimension (P = 0.02). Patients with normal ventricular internal systolic dimension (<4 cm) had a significantly longer time- to-first VT compared to those with abnormal LVIDs.
Table 1.
Baseline characteristics.
| ECHOCARDIOGRAPHIC PARAMETERS | MALES N = 474 |
FEMALES N = 117 |
|---|---|---|
| LEFT ATRIUM ABN: >4 cm | 180 (67.4%) | 35 (52.2%) |
| LVIDd ABN: >5.6 cm | 185 (70.6%) | 42 (65.6%) |
| LVIDs ABN: >4 cm | 187 (72.5%) | 49 (79.0%) |
| RWT ABN: ≥0.45 cm | 86 (34.3%) | 16 (25.4%) |
Table 2.
Echocardiographic parameters.
| ECHOCARDIOGRAPHIC PARAMETER | 12-MONTH % VT-FREE (95% CI) | LOG-RANK TEST P-VALUE | |
|---|---|---|---|
| Left Atrium (LA) | Abn | 86% (80%, 92%) | 0.20 |
| Nml | 87% (79%, 95%) | ||
| Relative Wall Thickness (RWT) | Abn | 88% (80%, 96%) | 0.22 |
| Nml | 86% (80%, 92%) | ||
| Left Ventricle in Diastole (LVIDd) | Abn | 86% (80%, 92%) | 0.70 |
| Nml | 88% (80%, 96%) | ||
| Left Ventricle in Systole (LVIDs) | Abn | 83% (77%, 89%) | 0.02** |
| Nml | 97% (93%, 100%*) |
Note:
significant value.
Abbreviations: Abn, abnormal; Nml, normal.
Survival Function
VT-free period
The following risk factors were individually found to be associated with time-to-first VT: age over 65 years (P = 0.02), use of diuretics (P = 0.03) or digitalis (P = 0.02) as medical therapy, having had coronary artery bypass grafting (CABG) surgery (P = 0.001), and having an abnormal LVIDs (P = 0.02). A backward selection algorithm was used to arrive at the final model for time-to-first VT. This more parsimonious model consists of LVIDs (P = 0.01) and CABG surgery (P = 0.01) as the most significant risk factors for a VT event (Table 3).
Table 3.
Backward selection algorithm for time-to-first VT.
| n = 591 | MALES N = 474 |
FEMALES N = 117 |
P-VALUE |
|---|---|---|---|
| Age more than 65 years | 378 (64.2%) | 211 (35.8%) | 0.21 |
| Hypertension | 412 (88.0%) | 102 (89.5%) | 0.87 |
| Diabetes | 118 (25.3%) | 40 (34.8%) | 0.06 |
| Valve replacement history | 164 (34.6%) | 35 (29.9%) | 0.38 |
| Coronary artery bypass surgery | 120 (25.5%) | 17 (14.8%) | 0.02 |
| Percutaneous coronary intervention | 52 (11.1%) | 6 (5.2%) | 0.06 |
| Syncope history | 63 (13.4%) | 14 (12%) | 0.88 |
| Myocardial infarction | 147 (31.2%) | 23 (20.2%) | 0.17 |
| A trial Fibrillation history | 79 (16.7%) | 17 (14.5%) | 0.67 |
Kaplan–Meier survival plots
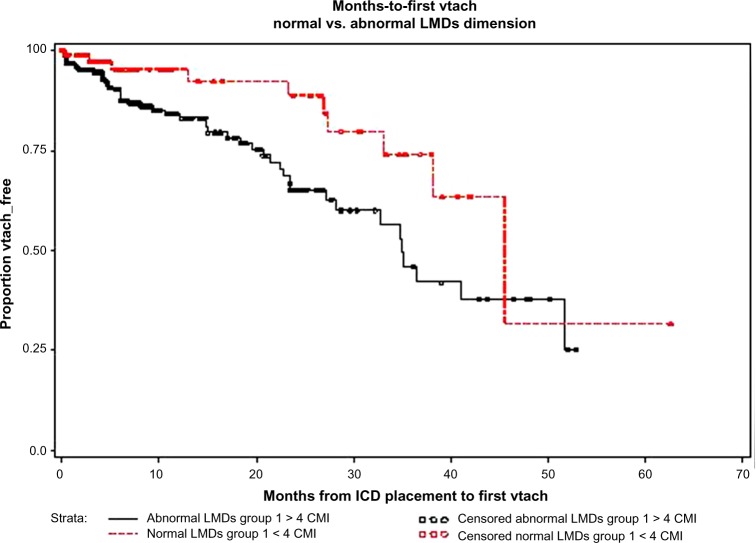
Figure 1 plots the proportion of VT-free subjects vs the number of months from initial ICD placement. The ordinate axis represents the proportion of subjects who have not had a VT episode. Censored observations (indicated by circles superimposed upon the hatched and solid lines) are those patients who have not experienced a VT as of last follow-up. These are patients with no episodes of VT recorded on their electrograms from the ICD as of their last clinic visit.
Figure 1.
LVIDs: survival function (VT-free period, Kaplan–Meier survival plot). Subjects in the abnormal LVIDs group (solid line) had, on average, a shorter time-to-first VT than those in the normal LVIDs group (hatched line).
Hazard ratio
The hazard ratio attributed to those who had an abnormal LVIDs vs those who did not was 2.5 [95% CI: (1.3, 5.0)]. This implies that subjects who had an abnormal LVIDs were 2.5 times more likely to have VT (and therefore an ICD discharge) than those with normal LVIDs (Table 3). Subjects who have had CABG surgery are 3.5 times more likely to have a VT than those who did not. Among subjects whose reason for ICD implantation was considered as a secondary prevention therapy, the following variables were individually associated with first VT: age >65 (P = 0.047), CABG surgery (P = 0.0005), digitalis (P = 0.017), diuretic (P = 0.02), lipid agent (P = 0.012), previous CHF (P = 0.03), and LIVDs (P = 0.0239). However, a backward selection algorithm using the Cox regression resulted in CABG surgery as the only factor significantly associated with longer time-to-first VT. [For the 77 patients whose ICD was implanted for primary prevention, age >65 (P = 0.02), history of AFIB (P = 0.004), and MADIT-II (P = 0.02) were individually associated with VT; however, a backward elimination algorithm showed that none of these three variables were significant].
Discussion
SCD most commonly results from cardiac arrest because of ventricular arrhythmias, primarily VF and VT usually in the setting of underlying structural heart disease with diminished LVEF. SCD accounts for approximately 15% of the total mortality in the United States and other industrialized countries, particularly prevalent among patients with heart failure. Despite the significant advances made over the past decade in the management of heart failure, it is estimated that about half of diagnosed heart failure patients die suddenly, most commonly as a result of fatal arrhythmias or further myocardial ischemic episodes.12–14 As a result of the high rate of SCD in these patients and among patients with documented myocardial arrhythmogenicity (evidenced by low LVEF, inducible arrhythmias during electrophysiology (EP) studies, or prior episodes of ventricular arrhythmia), the quantification of identifiable risk factors for the future development of fatal arrhythmias is essential.
While LVEF has proven to be a valuable echocardiographic parameter for determination of risk of ventricular arrhythmias, and therefore ICD eligibility, the question often arises of whether other parameters that are routinely assessed during echocardiography have the potential to serve as additional indicators of prognosis as well as predictors of future risk of SCD in patients deemed to be at risk of developing malignant ventricular arrhythmias. Despite the demonstrated efficacy of employing LVEF as a factor in determining ICD eligibility in most studies, the need for further quantifiable parameters is clear. Utilizing more varied parameters, in addition to ejection fraction, in determining ICD eligibility as well as for the optimization of possible antiarrhythmic therapy may have the potential to reduce the morbidity and mortality associated with the most common ventricular arrhythmias.
The importance of employing factors in addition to LVEF in structuring therapy for patients at risk of cardiac arrhythmias has been highlighted by several major clinical trials aimed at assessing the efficacy of ICD therapy. In the MADIT trial, for example, while patients with an LVEF below 26% demonstrated the greatest survival benefit from ICD implantation, those subjects with an LVEF between 26% and 35% did not demonstrate improved survival outcomes with an ICD compared with those who received conventional antiarrhythmic pharmacotherapy (thus, prompting the MADIT-II investigators to mandate an LVEF less than 30% as a prerequisite for entry into this later study).15 In the Multicenter Unsustained Tachycardia Trial (MUSTT), LVEF had a relatively low specificity in predicting SCD. The study, which in part specifically addressed the utility of LVEF as a predictor of the risk of SCD, demonstrated no significant difference in the number of patients with SCD attributed to arrhythmia in patients with an LVEF below 30% compared to those with an LVEF between 30% and 40%.16 Numerous other studies demonstrated similar results. In the European Autonomic Tone and Reflexes After Myocardial Infarction (ATRAMI) study, which recruited 1284 post-MI patients, less than half of the patients whose deaths were attributed to SCD in the 21-month follow-up period had an LVEF below 35%, although these results may be affected by the fact that there were significantly less patients in this study with LVEF below 35%.17,18
Such findings as those from MADIT-I, and the ATRAMI trials call into question the overutilization of LVEF as the sole indicator of potential benefit from ICD implantation and have led to attempts at identifying other parameters that can be useful for risk stratification. Given the finding of a relatively low specificity of LVEF in predicting the risk of sudden death, the MUSTT investigators identified other factors that were thought to have a high predictive value, including prior digitalis therapy, left bundle-branch block, and nonspecific intraventricular conduction delay.18,19 The ATRAMI trial demonstrated increased sensitivity in predicting the risk of SCD in post-MI patients by integrating LV systolic dysfunction/low LVEF with markers of depressed vagal activity, particularly heart rate variability and depressed baroreflex sensitivity.17 Despite the possibility of using such factors as additional markers of increased risk of ventricular arrhythmias, these variables are not yet currently employed in routine day-to-day practice.
While TTE is used to analyze myocardial function in patients with suspected cardiomyopathy, much of the data that are available from echocardiography often go unused in assessing risk of future adverse cardiac events. Our study aimed to correlate echocardiographic parameters other than LVEF with the risk of malignant ventricular arrhythmias following ICD implantation. Our data indicate that certain structural parameters, primarily LVIDs and LA size, may serve as surrogate markers in addition to LVEF to predict which patients may benefit from ICD therapy or to assist in alteration of ICD parameters or adjunctive antiarrhythmic pharmacotherapy in patients who already have an ICD.
Our data indicated that an LVIDs of greater than 4 cm was significantly associated with the occurrence of a VT in the post-ICD implantation period. Patients with LVIDs >4 cm were over twice more likely to experience VT than those patients with normal LV systolic dimensions (HR = 2.5). This finding is not entirely surprising given the established electromechanical defects and potential myocardial dyssynchrony associated with an enlarged left ventricle.20 Given our findings, LVIDs, as one of the parameters used to assess LV systolic function, may be used in combination with LVEF to enhance the sensitivity of echocardiography in predicting which patients may benefit most from ICD therapy. Long-term structural and neurohormonal changes lead to alterations in the cardiac microenvironment, which in turn results in enhanced arrhythmogenicity and is manifest as systolic dysfunction, which can be diagnosed via reduced LVEF and increased LVIDs.
Study Limitations
Our data were collected retrospectively, and therefore, the accuracy depends on the accuracy of the medical records and clinic charts. Some patients who are not currently followed up at our institution might have had episodes of VT after they were lost to follow-up, which could not be accounted for. Second, given the retrospective design, the presence of biases in the treatment for patients who are deemed to be at high risk for VT by the managing electrophysiologist cannot be ruled out with certainty. Third, not all ICD devices were implanted for primary prevention. Some patients may have been VT free longer after the initial implantation because of long-standing antiarrhythmic therapy. Finally, although our study had a large number of patients (586), because of the fact that this number was not based on a formal power calculation, the number of patients included in our study might have prevented some of the trends observed from reaching statistical significance. If echocardiographic parameters such as the ones mentioned were evaluated in more large-scale studies, and a more homogeneous group of subjects with more data on medication regimens and follow-up routines, the significance of this association could be explored further and elaboration of such relationships could alter targets for antiarrhythmic therapy.
Conclusion
The determination of echocardiographic parameters that can be used to reliably assess the risk of the development of life-threatening ventricular arrhythmias can theoretically improve outcomes by guiding alterations in either pharmacologic or electrophysiologic therapy and in risk stratification prior to consideration for ICD implantation. Certain echocardiographic parameters may impact survival in patients with future episodes of ventricular arrhythmias, and it is of concern which of these echocardiographic parameters can serve as predictors of electrical discharge from the ICD. While our study indicated that the main echocardiographic parameter associated with increased risk of VT was an increased LV dimension during systole, other factors that can serve as reliable predictors of future risk of ventricular arrhythmias are likely to be discovered with more wide-scale clinical trials. The addition of more variables to evaluate eligibility for ICD therapy and to alter the antiarrhythmic therapeutic approach is likely to improve outcomes in patients deemed to be at risk for developing malignant ventricular arrhythmias. Our study should provide the impetus for more large-scale studies evaluating the utility of readily available echocardiographic parameters as predictors of ventricular arrhythmias. Based on the likelihood of arrhythmia occurrence as assessed by specific echocardiographic parameter (such as LVIDs and LVEF), pharmacologic antiarrhythmic therapy and future programing of the ICD may be tailored to, respectively, prevent and terminate VT in addition to the earlier implantation of primary prevention, the ICD. The use of these parameters in conjunction with LVEF has the potential to improve outcomes in patients predisposed to malignant ventricular arrhythmias.
Footnotes
ACADEMIC EDITOR: Thomas E Vanhecke, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE). Provenance: the authors were invited to submit this paper.
Author Contributions
Conceived and designed the experiments: JNC, ANM, RJ. Analyzed the data: CS, JNM. Wrote the first draft of the manuscript: JNC, JNM. Contributed to the writing of the manuscript: ANM, CV, DF, RJ. Agree with manuscript results and conclusions: JNC, JNM, ANM, CS, CV, DF, RJ. Jointly developed the structure and arguments for the paper: JNC, JNM, ANM. Made critical revisions and approved final version: JNC, JNM, ANM, CS, CV, DF, RJ. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 2.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter – defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 3.Antiarrhythmics versus implantable defibrillators (AVID) Investigators A comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576–83. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 4.Kuck KH, Cappato R, Siebels J, Ruppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH) Circulation. 2000;102:748–54. doi: 10.1161/01.cir.102.7.748. [DOI] [PubMed] [Google Scholar]
- 5.Connolly SJ, Hallstrom AP, Cappato R, et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials: AVID, CASH and CIDS studies. Eur Heart J. 2000;21:2071–8. doi: 10.1053/euhj.2000.2476. [DOI] [PubMed] [Google Scholar]
- 6.Kusumoto FM, Calkins H, Boehmer J, et al. HRS/ACC/AHA expert consensus statement on the use of implantable cardioverter-defibrillator therapy in patients who are not included or not well represented in clinical trials. J Am Coll Cardiol. 2014;64(11):1143–77. doi: 10.1016/j.jacc.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Bhandari AK, Widerhorn J, Sager PT, et al. Prognostic significance of programmed ventricular stimulation in patients surviving complicated acute myocardial infarction: a prospective study. Am Heart J. 1992;124:87–96. doi: 10.1016/0002-8703(92)90924-k. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe H, Chinushi M, Sugiura H, et al. Unsuccessful internal defibrillation in Brugada syndrome: focus on refractoriness and ventricular fibrillation cycle length. J Cardiovasc Electrophysiol. 2005;16:262–6. doi: 10.1046/j.1540-8167.2005.40579.x. [DOI] [PubMed] [Google Scholar]
- 9.Zareba W, Moss AJ, Daubert JP, Hall WJ, Robinson JL, Andrews M. Implantable cardioverter defibrillator in high-risk long QT syndrome patients. J Cardiovasc Electrophysiol. 2003;14:337–41. doi: 10.1046/j.1540-8167.2003.02545.x. [DOI] [PubMed] [Google Scholar]
- 10.Begley DA, Mohiddin SA, Tripodi D, Winkler JB, Fananapazir L. Efficacy of implantable cardioverter defibrillator therapy for primary and secondary prevention of sudden cardiac death in hypertrophic cardiomyopathy. Pacing Clin Electrophysiol. 2003;26:1887–96. doi: 10.1046/j.1460-9592.2003.00285.x. [DOI] [PubMed] [Google Scholar]
- 11.Lang RM, Bierig M, Devereux RB, et al. Chamber Quantification Writing Group, American Society of Echocardiography’s Guidelines and Standards Committee, European Association of Echocardiography Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography. J Am Soc Echocardiogr. 2005;18(12):1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Kannel WB, Plehn JF, Cupples LA. Cardiac failure and sudden death in the Framingham Study. Am Heart J. 1988;115:869–75. doi: 10.1016/0002-8703(88)90891-5. [DOI] [PubMed] [Google Scholar]
- 13.Luu M, Stevenson WG, Stevenson LW, Baron K, Walden J. Diverse mechanisms of unexpected cardiac arrest in advanced heart failure. Circulation. 1989;80:1675–80. doi: 10.1161/01.cir.80.6.1675. [DOI] [PubMed] [Google Scholar]
- 14.Faggiano P, d’Aloia A, Gualeni A, Gardinia A, Giordano A. Mechanisms and immediate outcome of in-hospital cardiac arrest in patients with advanced heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2001;87:655–7. A10–1. doi: 10.1016/s0002-9149(00)01450-8. [DOI] [PubMed] [Google Scholar]
- 15.Moss AJ, Fadl Y, Zareba W, Cannom DS, Hall WJ, The Multicenter Automatic Defibrillator Implantation Trial Research Group Survival benefit with an implanted defibrillator in relation to mortality risk in chronic coronary heart disease. Am J Cardiol. 2001;88:516–20. doi: 10.1016/s0002-9149(01)01729-5. [DOI] [PubMed] [Google Scholar]
- 16.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. Multicenter unsustained tachycardia trial investigators. A randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med. 1999;341:1882–90. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 17.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–84. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 18.Goldberger Z, Lampert R. Implantable cardioverter-defibrillators: expanding indications and technologies. JAMA. 2006;295(7):809–18. doi: 10.1001/jama.295.7.809. [DOI] [PubMed] [Google Scholar]
- 19.Buxton AE, Lee KL, Hafley GE, et al. A simple model using the MUSTT database can stratify total mortality and sudden death risk of coronary disease patients. J Am Coll Cardiol. 2004;43:425A. [Google Scholar]
- 20.Yu C-M, Lin H, Zhang Q, Sanderson JE. High prevalence of left ventricular systolic and diastolic asynchrony in patients with congestive heart failure and normal QRS duration. Heart. 2003;89:54–60. doi: 10.1136/heart.89.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]