Abstract
Mucopolysaccharidosis type VII (MPS VII, Sly syndrome) is a very rare lysosomal storage disease caused by a deficiency of the enzyme β-glucuronidase (GUS), which is required for the degradation of three glycosaminoglycans (GAGs): dermatan sulfate, heparan sulfate and chondroitin sulfate. Progressive accumulation of these GAGs in lysosomes leads to increasing dysfunction in numerous tissues and organs. Enzyme replacement therapy (ERT) has been used successfully for other MPS disorders, but there is no approved treatment for MPS VII. Here we describe the first human treatment with recombinant human GUS (rhGUS), an investigational therapy for MPS VII, in a 12-year old boy with advanced stage MPS VII. Despite a tracheostomy, nocturnal continuous positive airway pressure, and oxygen therapy, significant pulmonary restriction and obstruction led to oxygen dependence and end-tidal carbon dioxide (ETCO2) levels in the 60-80 mmHg range, eventually approaching respiratory failure (ETCO2 of 100 mmHg) and the need for full-time ventilation. Since no additional medical measures could improve his function, we implemented experimental ERT by infusing rhGUS at 2 mg/kg over 4 hours every 2 weeks for 24 weeks. Safety was evaluated by standard assessments and observance for any infusion associated reactions (IARs). Urinary GAG (uGAG) levels, pulmonary function, oxygen dependence, CO2 levels, cardiac valve function, liver and spleen size, and growth velocity were assessed to evaluate response to therapy. rhGUS infusions were well tolerated. No serious adverse events (SAEs) or IARs were observed. After initiation of rhGUS infusions, the patient's uGAG excretion decreased by more than 50%. Liver and spleen size were reduced within 2 weeks of the first infusion and reached normal size by 24 weeks. Pulmonary function appeared to improve during the course of treatment based on reduced changes in ETCO2 after off-ventilator challenges and a reduced oxygen requirement. The patient regained the ability to eat orally, gained weight, and his energy and activity levels increased. Over 24 weeks, treatment with every-other-week infusions of rhGUS was well tolerated with no SAEs, IARs, or hypersensitivity reactions and was associated with measurable improvement in objective clinical measures and quality of life.
Keywords: mucopolysaccharidosis type VII, enzyme replacement therapy, pulmonary function, physician global impression of change, hepatosplenomegaly, Sly syndrome
1.0 Introduction
Mucopolysaccharidosis type VII (MPS VII), also known as Sly Syndrome, is a very rare form of MPS characterized by a deficiency of the lysosomal enzyme β-glucuronidase (EC 3.2.1.31), which is required for the degradation of three glycosaminoglycans (GAGs): dermatan sulfate, heparan sulfate, and chondroitin sulfate [1-4]. Progressive accumulation of these GAGs in lysosomes leads to increasing dysfunction in numerous tissues and organs. MPS VII symptoms are varied and may include abnormally coarse facies, hepatosplenomegaly, pulmonary disease, cardiovascular complications, joint stiffness, short stature, and skeletal disease known as dysostosis multiplex [1]. Developmental delay may be present in more severely affected MPS VII patients.
No treatments are currently approved for MPS VII. Enzyme replacement therapy (ERT) has been used successfully in the treatment of other lysosomal storage disorders in humans including MPS I, MPS II, MPS IVA, and MPS VI [5-9]. In mouse models of MPS VII, ERT with β-glucuronidase has been shown to reduce lysosomal storage of GAG and improve behavior, auditory function, and skeletal dysplasia [10-12].
This report describes the results of the first human treatment with recombinant human β-glucuronidase (rhGUS), an investigational therapy, in a 12-year-old child with advanced stage MPS VII.
2.0 Materials and methods
2.1 Case description
The patient was a 12-year-old boy with advanced stage MPS VII who was born at 35 weeks gestation with hydrops fetalis and hepatosplenomegaly. At 18 months of age, the patient presented with severe cord compression, and diagnosis of MPS VII by leukocyte and fibroblast assay was made by a consulting geneticist (JF) at the time of a required cervical fusion surgery. No β-glucuronidase enzyme activity was detected in fibroblasts and negligible activity of 0.9 nmol/mg protein/hour (reference range, 250.5-883.1) was detected in leukocytes. A mutation analysis in 2014 identified two mutations: S485F and W288L. Neither mutation has been previously reported in the literature to our knowledge.
Over the ensuing years, the patient's MPS VII disease progressed steadily. The patient had learned to walk and attended school, but he lost the ability to walk in 2010 and needed a tracheostomy due to upper airway obstruction. The patient had considerable progressive heart valve disease and an enlarging liver and spleen, both of which compounded his progressively worsening pulmonary status. In the year before rhGUS treatment, the patient was hospitalized several times for respiratory failure and elevated end-tidal carbon dioxide (ETCO2) levels of 80 mmHg or more. In September 2013, the patient was admitted again for pulmonary failure. By October 2013, the patient had progressively worsening pulmonary function leading to oxygen dependence and CO2 retention, and was close to pulmonary failure. Management of this patient's poor pulmonary function using maximally available treatment including tracheostomy, oxygen therapy, and full-time ventilation did not sufficiently support his pulmonary function and no additional medical measures could improve his pulmonary function. The patient was somnolent, relatively inactive during the day due to fatigue, and had stopped attending school. He took all feeds via gastrostomy tube (G tube). He was not considered likely to survive much longer if no new intervention was provided.
An emergency request for compassionate use of investigational rhGUS therapy was made and granted by the US Food and Drug Administration (FDA), and investigational rhGUS was provided by the drug developer. The patient and his parents provided informed consent to the treatment with rhGUS after a discussion of the potential risks and benefits of treatment. After consenting, the patient was evaluated for clinical condition using standard clinical assessments appropriate for his condition. Samples of urine and blood were taken to assess his baseline biochemical condition and to provide control specimens for evaluation of any immune response to rhGUS.
2.2 Treatment and assessments
The patient was treated by intravenous (IV) infusions of 2 mg/kg of rhGUS (Ultragenyx Pharmaceutical Inc., Novato, CA) administered over approximately 4 hours every 2 weeks, for 24 weeks. At 6 weeks, 12 weeks, and 24 weeks, the patient was evaluated to determine whether his condition and the benefits and risks compelled continued treatment with rhGUS.
Safety was evaluated by assessing for any adverse events (AEs) during exposure to rhGUS throughout the study by clinical laboratory measures and by noting the number and types of infusion associated reactions (IARs). All AEs occurring from the onset of the infusion to within four hours following the end of the infusion were considered as IARs. A preliminary assessment of formation of antibodies to rhGUS was performed using a standard bridging enzyme-linked immunosorbent assay (ELISA). Safety events were recorded in the patient's medical record.
Total urinary GAG (uGAG) excretion (normalized to urinary creatinine concentration) was evaluated in first morning void urine at baseline and weeks 2, 4, 6, 8, 10, 12, 18, and 24 using the nonreducing ends (NRE) method (ARUP Laboratories, Salt Lake City, Utah, USA) [13]. Other measures of efficacy were evaluated at baseline and weeks 12 and 24. These measures included pulmonary function assessed by CO2 retention level (ETCO2) and pulse oximetry; cardiac mass evaluated by echocardiogram and scored as a z score relative to normal ventricular mass; liver and spleen size evaluated by ultrasound and clinical exam; weight; length; and a physician global impression of change scale (PGI-C). To score the PGI-C, the physician caring for the patient provided a global assessment of change using a 7-point scale ranging from -3 (significant worsening) to +3 (significant improvement) [14]. The overall score was based on reported changes in a list of disease-specific abnormalities projected to respond to treatment. An institutional review board approved the study. This report provides results from the first 24 weeks of treatment and the study is ongoing.
3.0 Results
3.1 Patient History
The MPS VII patient presented with progressive worsening of pulmonary insufficiency with worsening CO2 retention of 80 mmHg or higher that was not sufficiently supported by maximally available medical treatment. Based on his clinical condition and progression at that time, ERT with investigational rhGUS represented this patient's only available option for potential improvement. Thus, an emergency request for compassionate use of investigational rhGUS therapy was made and granted by the FDA and investigational rhGUS was provided by the drug developer.
3.2 rhGUS Infusions
The patient was treated with IV infusion of 2 mg/kg of rhGUS every other week. A total of 12 infusions were received with one missed infusion at week 16 due to an intercurrent illness of respiratory infection with fever (Table 1). At week 24, the total dose administered was increased from 50 mg to 60 mg to account for an increase in the patient's weight (see section 3.4.5).
Table 1. rhGUS infusions administered over 24 weeks.
| Infusion | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | -- | 9 | 10 | 11 | 12 |
|
|
|||||||||||||
| Week | Baseline | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | 22 | 24 |
|
| |||||||||||||
|
Dose (2 mg/kg) |
50 mg | 50 mg | 50 mg | 50 mg | 50 mg | 50 mg | 50 mg | 50 mg | 0 mg | 50 mg | 50 mg | 50 mg | 60 mg |
At 30 to 60 minutes before each infusion, the patient was pretreated with an antihistamine medication, cetirizine hydrochloride, 7.5 mg per G tube. Within 24 hours after the first and second infusions, the patient experienced a non-pruritic erythematous rash on his chest that resolved or improved with hydroxyzine administration. The hydroxyzine became a routine post-infusion prophylactic for 24 hours after the infusion beginning with the third infusion. Post infusion hydroxyzine was ended after Week 32 without further hive-like rash observed. Initially, no antipyretic medication was provided but, beginning on the eighth infusion, ibuprofen (200 mg) was also administered prophylactically. The addition was due to a fever experienced after an infusion, although the fever was later determined to be due to an intercurrent illness.
After the first 4 infusions, some fatigue was noted for the day after the infusion. Based on experiences with other MPS diseases, it was speculated that fluid overload from the saline load of the infusion may be a problem due to the patient's advanced heart valve disease. Beginning with the fifth infusion, the patient received a single 10 mg dose of diuretic (furosemide) per G tube after each infusion. The diuretic reduced his fatigue the day after infusions and was therefore continued prophylactically after subsequent infusions.
3.3 Safety
Adverse events occurring during 24 weeks of study that were considered possibly related to rhGUS treatment are shown in Table 2.
Table 2. Adverse Events Considered Possibly Related to rhGUS Treatment.
| Week | Infusion | Time from Last Infusion to Start of AE | Duration of AE | Adverse Event | CTCAE Severitya |
|---|---|---|---|---|---|
| Baseline | 1 | 1 day | 1 day | Non pruritic erythematous rash | 1 |
| Baseline | 1 | 5 days | 1 day | Coarse rales, non-productive cough and requires suction | 1 |
| 4 | 3 | 1 day | 7 days | Increased ETCO2 | 1 |
| 6 | 4 | 7 days | 1 day | Increased ETCO2 | 1 |
| 8 | 5 | same day | 1 day | Increased ETCO2 | 1 |
| 8 | 5 | 1 day | 2 days | Desat, Fever Increased secretions | 1 |
| 12 | 7 | 1 day | 1 day | Elevated temp and heart rate | 1 |
| 12 | 7 | 1 day | 4 days | Blood-tinged secretions & Desat | 2 |
| 14 | 8 | 11 days | 2 days | Elevated temp and heart rate | 1 |
| 14 | 8 | 11 days | 2 days | Increased Secretions and Desat | 2 |
| 16 | - | 16 days | 3 days | Increased Secretions and Desat | 2 |
| 22 | 11 | 10 days | 1 day | Increased ETCO2 and emesis | 1 |
| 24 | 12 | 1 day | 4 days | Fatigue | 1 |
| 24 | 12 | 2 days | <1 day | Increased Temp | 1 |
Common Terminology Criteria for Adverse Events (CTCAE) Severity: 1=Mild, 2= Moderate, 3=Severe/undesirable, 4=Life threatening/ debilitating, 5=Death; AE, adverse event; ETCO2, end-tidal carbon dioxide; desat, desaturation; temp, temperature
No serious adverse events (SAEs) or hospitalizations and no IARs or hypersensitivity reactions were observed during the 24 weeks of treatment. Serum was collected for preliminary assessment of antibodies against rhGUS by ELISA. Background signal was detected in baseline samples, but no increase in signal was observed at 12 or 24 weeks. The assay is still being optimized.
3.4 Efficacy
3.4.1 uGAG Levels as a measure of lysosomal storage
Experience in 4 MPS diseases and prior work in animal models suggests that uGAG excretion corrected for creatinine concentration can provide a reasonable assessment of lysosomal storage in MPS diseases. At baseline, the patient's uGAG level as measured by the NRE method was 2.36 mg/mmol creatinine. Urinary GAG levels decreased rapidly upon treatment with rhGUS with a reduction of 59.1% from baseline observed at 2 weeks (Figure 1). A maximum reduction in uGAG of 74.6% from baseline was observed at 6 weeks; levels stabilized thereafter, with reductions of approximately 55%-65% from baseline.
Figure 1. Urinary GAG During Infusions With rhGUS.
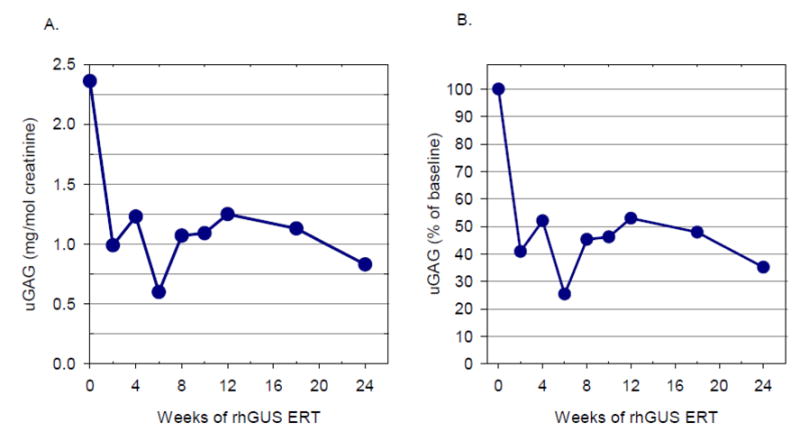
Urinary GAG as measured by the NRE method over 24 weeks of study is expressed as absolute levels (mg/mol creatinine) (A) and relative percent of baseline (B)
3.4.2 Pulmonary Function
Prior to his initial infusion with rhGUS, the patient had substantial CO2 retention as measured by ETCO2 testing and had pulmonary insufficiency bordering on pulmonary failure. He had begun receiving continuous ventilator support via his tracheostomy. After his second hospitalization for pulmonary distress, the ventilator synchronized intermittent mandatory ventilation (SIMV) rate was set at 18; it had been reduced to a rate of 12 but attempts to further decrease the rate to 10 were unsuccessful, and the patient could not tolerate 1-hour periods off the ventilator without ETCO2 levels rising to more than 80 mmHg. At the time of the initial rhGUS infusion, the patient's ventilator settings were SIMV rate 12, tidal volume 200, pressure support 10, and fraction of inspired oxygen (FIO2) 35%. On this setting, his ETCO2 ranged from the high 40s to low 50s mmHg.
Following rhGUS treatment, pulmonary function appeared to improve as the patient's liver and spleen size declined rapidly after a single dose, and then it reached a plateau. After 13 weeks of treatment (7 infusions), the patient was challenged with 1-hour periods off the ventilator. Pre- and post-challenge ETCO2 levels are shown in Figure 2. Initially, the patient had large increases (10 mmHg or more) in ETCO2 after 13-16 weeks of treatment and could not tolerate being off the ventilator. After 20 weeks of treatment, significantly smaller increases or no changes in post-challenge ETCO2 levels wereobserved. In addition, after 24 weeks of treatment, the patient tolerated periods of 80 minutes 2 times per day for total of 160 minutes per day off the ventilator without an increase in ETCO2 from an initial 53 mmHg. By week 24, supplemental oxygen had been successfully weaned to 28% from the previously noted 35% oxygen requirement.
Figure 2. Comparison in ETCO2 Before and After the Off-Ventilator Challenge.
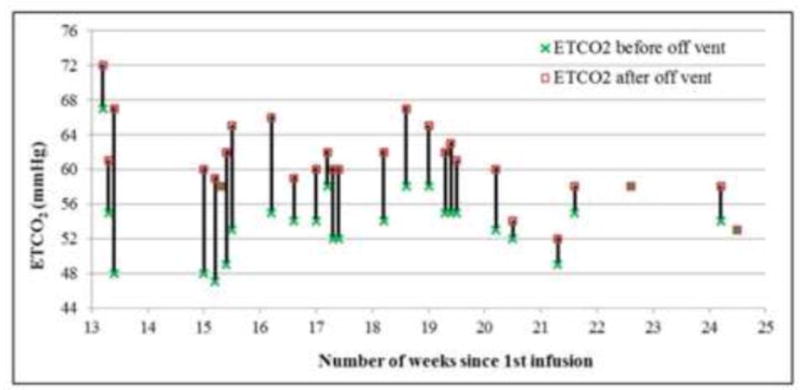
Off-ventilator challenges began after 13 weeks of rhGUS treatment. Changes in ETCO2 (in mmHg) before and after the off-ventilator challenge at each timepoint measured are shown.
3.4.3 Cardiac function
The patient underwent a transthoracic echocardiogram approximately 2 months before starting rhGUS therapy; this is considered the baseline cardiac measure for this study. At that time, there was mild mitral valve regurgitation, abnormal aortic valve with insufficiency, and a dilated aortic root. Hyperdynamic left ventricular shortening fraction, asymmetric septal hypertrophy, and diastolic dysfunction were observed; there was no pericardial effusion. Left ventricular (LV) mass was measured at 141.38 g and a LV mass index of 150.36 g/height2.7. As noted in section 3.2, these valve and heart function problems were sufficient to provide some difficulty with fluid overload after the infusions, necessitating the addition of furosemide treatment post infusion, which helped reduce post-infusion fatigue. A repeat transthoracic echocardiogram at week 14 prior to the patient's eighth infusion revealed no changes. LV mass and LV mass index were not assessed at week 14.
A baseline electrocardiogram (ECG) showed the QRS axis to be 43 degrees and ST at a rate of 123 beats per minute (bpm). Bi-atrial and left ventricular hypertrophy were present but there were no important ST-T changes and all intervals were normal. A repeat ECG at Week 14 showed the QRS axis to be 68 degrees and normal sinus rhythm of 105 bpm. No atrial hypertrophy was present; in addition, there were no ST-T changes and all intervals were normal. Possible biventricular hypertrophy was still noted.
3.4.4 Hepatosplenomegaly
The size of the patient's liver and spleen was estimated clinically and by using ultrasound but an accurate estimate of volume could not be obtained by ultrasound due to unusual anatomy. By clinical examination at baseline, his liver size was extremely large at 2 cm below the umbilicus and his spleen tip extended into his groin (Table 3). At 2 weeks after the first infusion, both his liver and spleen size had decreased substantially. By 24 weeks, liver and spleen size appeared normal, a finding confirmed by ultrasound.
Table 3. Liver and Spleen Size by Clinical Examination.
| Weeks | Liver | Spleen |
|---|---|---|
| Pre-treatment | ∼2 cm below umbilicus | Extended into groin |
| 2 | ∼1 cm above umbilicus | At umbilicus |
| 8 | ∼1 cm above umbilicus | At umbilicus |
| 12 | Above umbilicus | Above umbilicus |
| 24 | 3.5 cm above umbilicus (2cm below costal margin) |
Palpable spleen tip |
3.4.5 Growth
Prior to rhGUS infusions, the patient had limited interest in pleasure feedings and was aspirating food. Pleasure feedings were done by the patient's mother generally after regular nourishment through his G tube.
After infusions, the patient's appetite increased and the patient asked for more food from the staff. A swallow exam performed after 19 weeks of treatment showed the patient was no longer aspirating food. As significant, unexpected weight gain was also noted, his supplemental gastrostomy feedings were decreased to twice per day prior to breakfast and lunch only, leading to some loss of weight in the last period as noted in Figure 3. Supplements were changed in relationship to new dietary changes. The patient continues to tolerate oral feedings with supplemental gastrostomy intake. In July 2014, after approximately 38 weeks of treatment, a repeat modified barium study was done. While he continues to be a significant aspiration risk with thin liquids, he was noted to safely tolerate thickened liquids, solids, and purees allowing expansion of his dietary intake.
Figure 3. Weight Growth During Treatment with rhGUS.
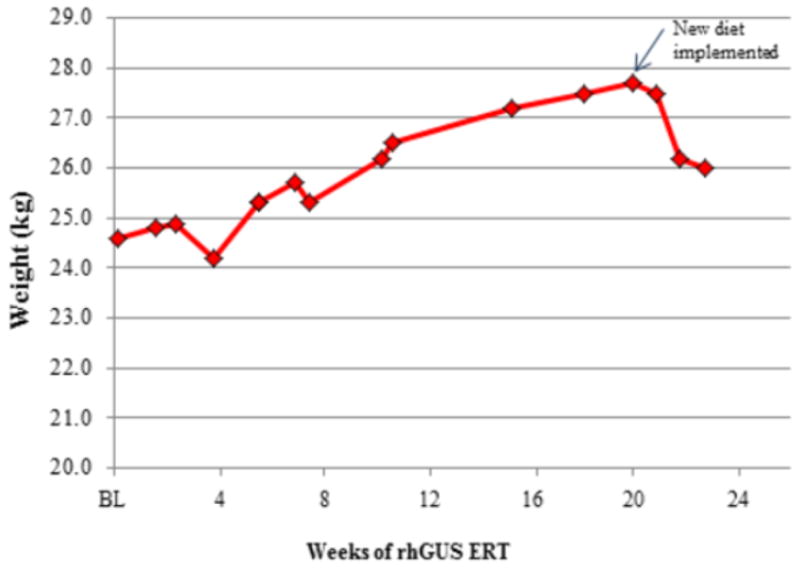
Patient weight as measured in kg over 24 weeks of study is shown. Supplemental gastrostomy feedings were reduced after 19 weeks leading to reduction in weight gained.
Patient length measured as standing height was difficult to obtain due to lack of walking and weight bearing. Supine length was 110 cm at the start of infusions and did not change during 24 weeks of study.
3.4.6 Physician Global Impression of Change
The patient's primary physician noted general improvements in health after initiation of rhGUS infusions. Key findings included less fatigue and increased stamina; increased school attendance; and independent manipulation of his wheelchair, which the patient had not done for the previous two years.
For the PGI-C, a set of clinical problems were identified at baseline and assessed at 12 and 24 weeks using a 7-point score (Table 4). At both weeks 12 and 24, the patient had an overall score of +2, indicating moderate improvement overall.
Table 4. PGI-C Score as assessed by characterization of specific MPS clinical findings.
| BASELINE | ||||
|---|---|---|---|---|
| MPS PHYSICAL FINDINGS | Present | Not Present | Week 12 | Week 24 |
| Short stature/slow growth rate | Y | 0 | 0 | |
| Limited visual acuity/difficulty reading plain text | Y | 0 | 0 | |
| Frequent infections (ear, respiratory, sinus) | Y | 0 | 0 | |
| Limited endurance/fatigue/lack of “energy”/malaise | Y | 1 | 1 | |
| Short of breath/difficulty breathing on exertion | Y | 1 | 1 | |
| Diminished pulmonary function | Y | 1 | 1 | |
| Respiratory support needed (tracheostomy, BiPAP, CPAP, supplemental O2) | Y | 1 | 1 | |
| Diminished walking ability (joint stiffness, contractures) | Y | 0 | 0 | |
| Assistive walking devices needed (walker, AFOs, wheelchair etc.) | Y | 0 | 0 | |
| Difficulty with transfers (up from chair or floor, in/outof bed, tub, car) | Y | 0 | 0 | |
| Difficulty bending over due to enlarged abdomen | Y | 1 | 1 | |
| Poor appetite (limited desire and difficulty eating) | Y | 2 | 1 | |
| Overall Score | 2 | 2 | ||
| Entry Codes: | |
| N = New Finding | 0= No Change |
| -1 = Mild Worsening | +1 = Mild Improvement |
| -2 = Moderate Worsening | +2 = Moderate Improvement |
| -3 = Significant Worsening | +3 = Significant Improvement |
4.0 Discussion
This report presents findings from the first-in-human treatment with investigational rhGUS ERT in a 12-year-old patient with MPS VII. Over 24 weeks, treatment with every-other-week infusions of rhGUS was well tolerated with no SAEs, IARs, or hypersensitivity reactions and with measurable improvement in objective clinical measures and quality of life.
After initiation of rhGUS infusions, the patient's liver and spleen rapidly reduced in size, and his urinary GAG excretion decreased by more than 50%, a reduction similar to that observed in studies of ERT for other MPS disorders [7, 8]. Pulmonary function appeared to improve somewhat during the course of treatment. After 16 weeks of rhGUS therapy, the patient could begin to tolerate brief off-ventilator challenges with smaller increases in ETCO2 observed as the study went on, but ventilator support was not withdrawn. No significant changes in cardiac function were noted.
rhGUS treatment significantly enhanced the patient's quality of life. Since the initiation of the infusion protocol, the patient showed marked improvement in his energy level as manifested by his ability to participate in school and recreational activities, and decreased daytime sleeping. He also recovered some expanded use of his upper extremities with the ability to pronate and point, allowing use of electronic devices for entertainment and remote interaction with his mother and manipulation of his wheelchair. The patient also became more interested in pleasure feeding. Whereas he was aspirating food at the beginning of the study, the return of a safe swallow reflex allowed him to begin oral feedings again, and by 24 weeks his nutrition was significantly supported by his oral intake. The hospital staff noted the patient's increased appetite and described him as being “constantly hungry”.
rhGUS infusions were generally well tolerated. The patient did not have any hypersensitivity reactions or IARs and no immune response has yet been documented. Observations of rash and fluid overload after the first few infusions led to prophylactic implementation of antihistamine and furosemide with subsequent infusions, which minimized these effects.
Overall, the patient has improved on rhGUS ERT with a reduction in lysosomal storage and increased energy and activity levels. He is able to eat orally and his pulmonary function has improved clinically, but he still remains a child with significant health issues. The rhGUS infusions have been well tolerated and study is ongoing to assess the outcomes of further treatment.
Highlights.
rhGUS is an experimental enzyme replacement therapy for mucopolysaccharidosis type VII
We report the first human use of rhGUS in a patient with advanced stage MPS VII
rhGUS infusions were generally well tolerated
Indicators of lysosomal storage decreased within 2 weeks of treatment initiation
Positive treatment effects also observed for pulmonary function and quality of life
Acknowledgments
The study is sponsored by the Cohen Children's Medical Center, with funding from the investigational product's developer, Ultragenyx Pharmaceutical Inc. We thank Melita Dvorak-Ewell, PhD, of Ultragenyx for analysis and preparation of the graphs and figures and Holly B Zoog, PhD, of Ultragenyx for providing medical writing support.
Dr Fox has received research grants from Ultragenyx Pharmaceutical Inc.
Abbreviations
- AE
adverse event
- bpm
beats per minute
- CO2
carbon dioxide
- CPAP
continuous positive airway pressure
- CTCAE
common terminology criteria for adverse events
- Desat
desaturation
- ECG
electrocardiogram
- ECHO
echocardiogram
- ELISA
enzyme-linked immunosorbent assay
- ERT
enzyme replacement therapy
- ETCO2
end-tidal carbon dioxide
- FDA
Food and Drug Administration
- FIO2
fraction of inspired oxygen
- GAG
glycosaminoglycans
- G tube
gastrostomy tube
- GUS
glucuronidase
- HCl
hydrochloride
- IAR
infusion associated reaction
- kg
kilogram
- LV
left ventricle
- mg
milligram
- mmHg
millimeters of mercury
- mmol
millimole
- MPS
mucopolysaccharidosis
- MPS I
mucopolysaccharidosis type I
- MPS II
mucopolysaccharidosis type II
- MPS IVA
mucopolysaccharidosis type IVA, Morquio A Syndrome
- MPS VI
mucopolysaccharidosis type VI
- MPS VII
mucopolysaccharidosis type VII
- NRE
non-reducing ends
- PGI-C
physician's global impression of change
- rhGUS
recombinant human glucuronidase
- SAE
serious adverse event
- SIMV
synchronized intermittent mandatory ventilation
- Temp
temperature
- uGAG
urinary glycosaminoglycan
- US
United States
Footnotes
Disclosures: Dr Volpe reports no conflicts of interest.
Ms Bullaro reports no conflicts of interest.
Dr Kakkis is an employee of and holds stock in Ultragenyx Pharmaceutical Inc., the developer of rhGUS. In addition, Dr. Kakkis has a patent filing on a form of recombinant human glucuronidase pending.
Dr Sly reports no financial conflicts. His institution, St Louis University licensed the cell line for producing rhGUS to Ultragenyx.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neufeld E, Muenzer J. The mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inhertited disease. McGraw-Hill, New York: 2001. [Google Scholar]
- 2.Sly WS, Quinton BA, McAlister WH, Rimoin DL. Beta glucuronidase deficiency: Report of clinical, radiologic, and biochemical features of a new mucopolysaccharidosis. J Pediatr. 1973;82:249–257. doi: 10.1016/s0022-3476(73)80162-3. [DOI] [PubMed] [Google Scholar]
- 3.Shimada T, Tomatsu S, Yasuda E, Mason RW, Mackenzie WG, Shibata Y, Kubaski F, Giugliani R, Yamaguchi S, Suzuki Y, Orii K, Orii T. Chondroitin 6-sulfate as a novel biomarker for mucopolysaccharidosis iva and vii. JIMD Rep. 2014 doi: 10.1007/8904_2014_311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogler C, Levy B, Kyle JW, Sly WS, Williamson J, Whyte MP. Mucopolysaccharidosis vii: Postmortem biochemical and pathological findings in a young adult with beta-glucuronidase deficiency. Mod Pathol. 1994;7:132–137. [PubMed] [Google Scholar]
- 5.Harmatz P, Giugliani R, Schwartz I, Guffon N, Teles EL, Miranda MC, Wraith JE, Beck M, Arash L, Scarpa M, Yu ZF, Wittes J, Berger KI, Newman MS, Lowe AM, Kakkis E, Swiedler SJ, M.V.P.S. Group Enzyme replacement therapy for mucopolysaccharidosis vi: A phase 3, randomized, double-blind, placebo-controlled, multinational study of recombinant human n-acetylgalactosamine 4-sulfatase (recombinant human arylsulfatase b or rhasb) and follow-on, open-label extension study. J Pediatr. 2006;148:533–539. doi: 10.1016/j.jpeds.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Kakkis ED, Muenzer J, Tiller GE, Waber L, Belmont J, Passage M, Izykowski B, Phillips J, Doroshow R, Walot I, Hoft R, Neufeld EF. Enzyme-replacement therapy in mucopolysaccharidosis i. N Engl J Med. 2001;344:182–188. doi: 10.1056/NEJM200101183440304. [DOI] [PubMed] [Google Scholar]
- 7.Muenzer J, Wraith JE, Beck M, Giugliani R, Harmatz P, Eng CM, Vellodi A, Martin R, Ramaswami U, Gucsavas-Calikoglu M, Vijayaraghavan S, Wendt S, Puga AC, Ulbrich B, Shinawi M, Cleary M, Piper D, Conway AM, Kimura A. A phase ii/iii clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis ii (hunter syndrome) Genet Med. 2006;8:465–473. doi: 10.1097/01.gim.0000232477.37660.fb. [DOI] [PubMed] [Google Scholar]
- 8.Wraith JE, Clarke LA, Beck M, Kolodny EH, Pastores GM, Muenzer J, Rapoport DM, Berger KI, Swiedler SJ, Kakkis ED, Braakman T, Chadbourne E, Walton-Bowen K, Cox GF. Enzyme replacement therapy for mucopolysaccharidosis i: A randomized, double-blinded, placebo-controlled, multinational study of recombinant human alpha-l-iduronidase (laronidase) J Pediatr. 2004;144:581–588. doi: 10.1016/j.jpeds.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 9.Hendriksz CJ, Burton B, Fleming TR, Harmatz P, Hughes D, Jones SA, Lin SP, Mengel E, Scarpa M, Valayannopoulos V, Giugliani R, S. Investigators. Slasor P, Lounsbury D, Dummer W. Efficacy and safety of enzyme replacement therapy with bmn 110 (elosulfase alfa) for morquio a syndrome (mucopolysaccharidosis iva): A phase 3 randomised placebo-controlled study. J Inherit Metab Dis. 2014 doi: 10.1007/s10545-014-9715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Connor LH, Erway LC, Vogler CA, Sly WS, Nicholes A, Grubb J, Holmberg SW, Levy B, Sands MS. Enzyme replacement therapy for murine mucopolysaccharidosis type vii leads to improvements in behavior and auditory function. J Clin Invest. 1998;101:1394–1400. doi: 10.1172/JCI1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogler C, Sands MS, Galvin N, Levy B, Thorpe C, Barker J, Sly WS. Murine mucopolysaccharidosis type vii: The impact of therapies on the clinical course and pathology in a murine model of lysosomal storage disease. J Inherit Metab Dis. 1998;21:575–586. doi: 10.1023/a:1005423222927. [DOI] [PubMed] [Google Scholar]
- 12.Vogler C, Sands MS, Levy B, Galvin N, Birkenmeier EH, Sly WS. Enzyme replacement with recombinant beta-glucuronidase in murine mucopolysaccharidosis type vii: Impact of therapy during the first six weeks of life on subsequent lysosomal storage, growth, and survival. Pediatr Res. 1996;39:1050–1054. doi: 10.1203/00006450-199606000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence R, Brown JR, Al-Mafraji K, Lamanna WC, Beitel JR, Boons GJ, Esko JD, Crawford BE. Disease-specific non-reducing end carbohydrate biomarkers for mucopolysaccharidoses. Nat Chem Biol. 2012;8:197–204. doi: 10.1038/nchembio.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guy W. The clinical global impression scale. In: Rush AJJ, First MB, Blacker D, editors. Handbook of psychiatric measures. American Psychiatric Publishing, Inc.; Washington, D.C: 2008. pp. 90–92. [Google Scholar]


