Figure 1. The electron transfer pathway.
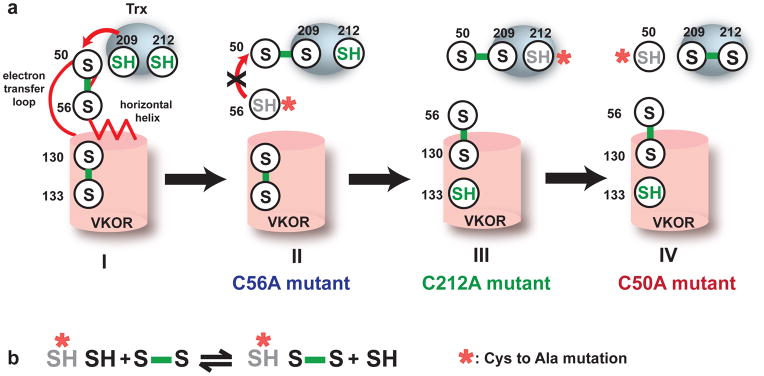
The electron transfer process can be followed by the changes of sulfhydryls (colored in green). a, State I: At this initial state, Cys209 and Cys212 at the active site of Trx domain have been reduced by nascent proteins. The Cys209 and Cys212 sulfhydryls will subsequently reduce the Cys50-Cys56 disulfide. State II: Cys209/Cys212 forms a mixed disulfide with Cys50/Cys56. The red arrow shows a possible backward reaction in which the Cys56 attacks the Cys50-Cys209 disulfide. A Cys56Ala mutation (red asterisk) directly prevents this backward attack (black cross) and stabilizes the Cys50-Cys209 disulfide. State III: Physical separation of Cys56 from Cys50-Cys209 disulfide may also prevent the backward attack. The free Cys56 can interact with Cys130/Cys133 at the active site of VKOR, leaving behind the Cys50/Cys209/Cys212. A Cys212Ala mutation (red asterisk) can stabilize this state. State IV: The free Cys50 reduces the Cys56-Cys130 disulfide. A Cys50Ala mutation prevents this attack and stabilizes the Cys56-Cys130 disulfide. b, The strategy of capturing electron-transfer intermediates. A single mutation (red asterisk) in a cysteine pair will generate a free thiol in the other cysteine, which subsequently reduce another cysteine pair and form an alternative disulfide.
