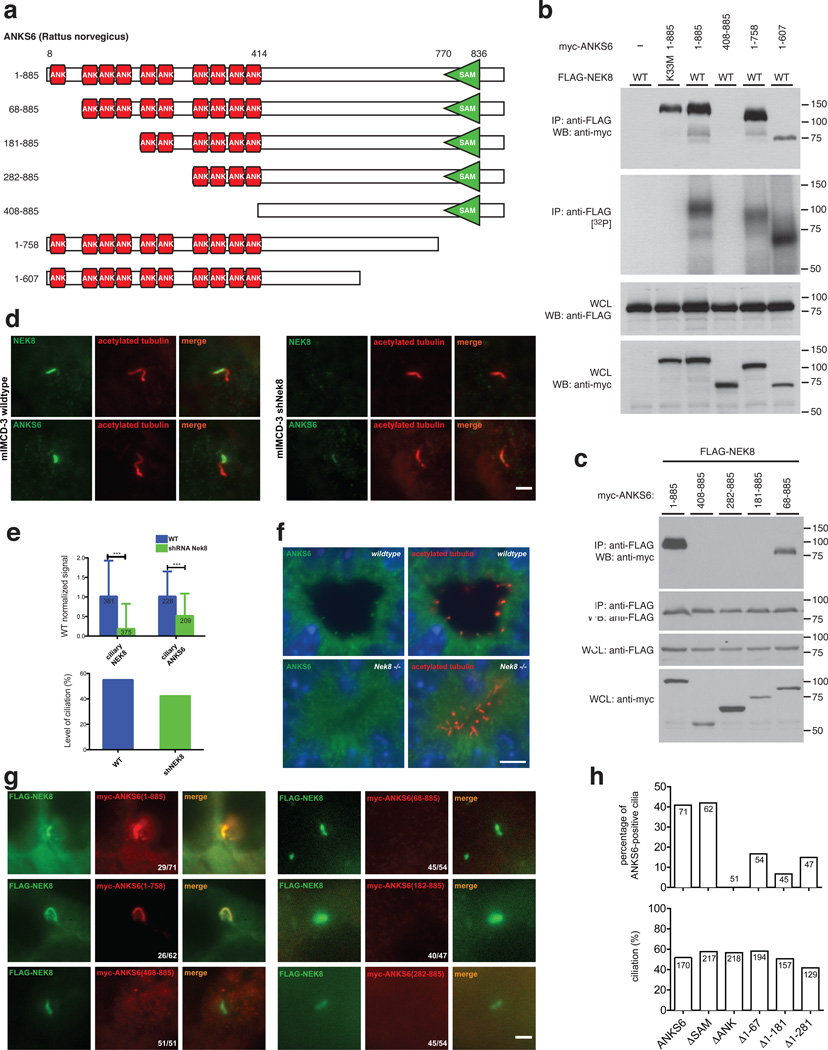
Figure 3. The ankyrin-repeat domain of ANKS6 is necessary for NEK8 binding and targeting of ANKS6 to the inversin compartment.
a – Different ANKS6 truncations were employed in IP/binding assays and IF. b – ANKS6 interacts with NEK8 through its ANK-repeat domain, while the SAM-domain is not essential for binding. ANKS6 truncations that interact with NEK8 are readily phosphorylated in complex with NEK8. c – A truncation variant of ANKS6 lacking the first ANK-repeat binds to NEK8, while truncation of the first four ANK-repeats completely abrogates binding. d, e – shRNA-mediated targeted knockdown of NEK8 significantly reduces the ciliary ANKS6-signal. IF signal intensities were compared between wildtype IMCD cells and IMCDshNek8 cells, for both anti-NEK8 and anti-ANKS6 signals (scale bar: 2 µm). The number of individual cilia analyzed is indicated in the respective bar. Differences were highly significant by Student’s t-test with p<0.01 (upper bar graph). The total level of ciliation is only mildly affected by Nek8 knockdown (lower bar graph; percentage of numeric counts of ciliated cells over total cells). f – Fluorescence immunohistochemistry on kidney sections of wildtype (upper panels) and Nek8−/− mice (lower panels), employing antibodies against ANKS6 (green) and acetylated tubulin (red). Positive ANKS6 signals at the ciliary base can only be identified in the wildtype, not in Nek8−/− cilia (scale bar: 5 µm). g, h – Coexpression of myc-tagged ANKS6 truncation variants and FLAG-tagged wildtype NEK8 demonstrates the importance of the ANK-repeat domain for NEK8-binding and consecutive ciliary targeting of ANKS6 (scale bar: 2 µm; upper bar graph: percentage of numeric counts of ANKS6-positive cilia over total cilia; lower bar graph: percentual level of ciliation; the number of cells counted is indicated in each bar).