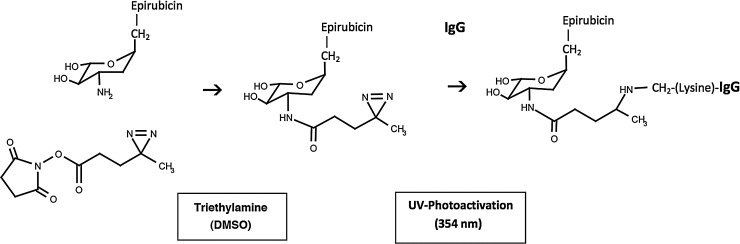
FIG. 1.
Schematic illustration of the chemical reactions involved in the synthesis of epirubicin-(C3-amide)-[anti-HER2/neu]. (Phase-I) creation of a covalent amide bond at the C3 monoamine of epirubicin and the ester group of succinimidyl 4,4-azipentanoate resulting in the creation of a covalent UV-photoactivated epirubicin-(C3-amide) intermediate accompanied by the liberation of the succinimide “leaving” complex; (Phase-II) creation of a covalent bond between the UV-photoactivated epirubicin-(C3-amide) intermediate and the ɛ-amine of lysine residues within the amino acid sequence of anti-HER2/neu monoclonal immunoglobulin initiated by photoactivation (UV 354 nm).