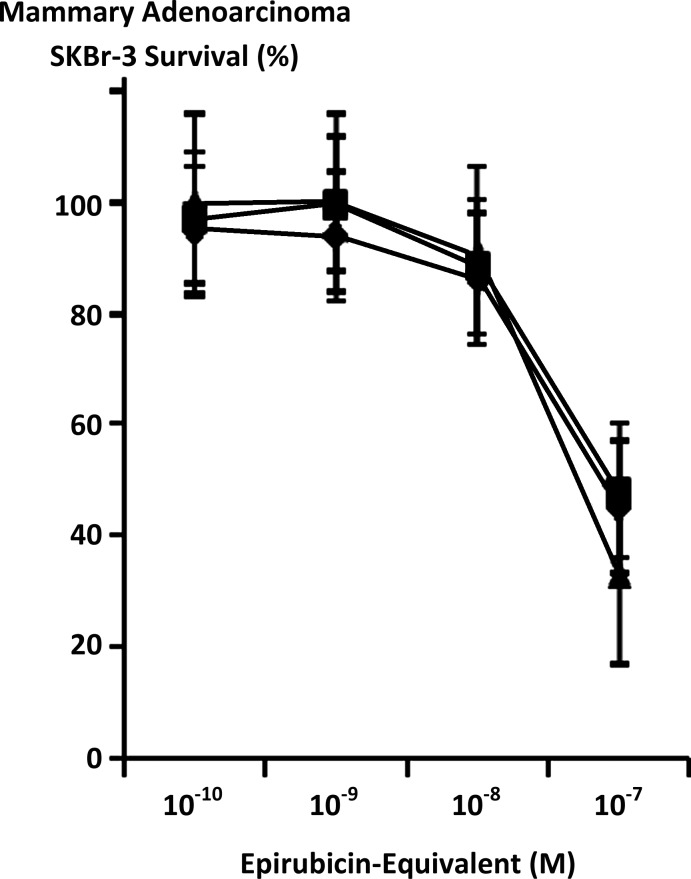
FIG. 6.
Relative cytotoxic anti-neoplastic potency of covalent epirubicin-[anti-HER2/neu] immunochemotherapeutics against chemotherapeutic-resistant mammary adenocarcinoma. (▴) epirubicin-(C3-amide)-[anti-HER2/neu] synthesized utilizing a UV-photoactivated epirubicin-(C3-amide) intermediate; (♦) epirubicin-(C13-imino)-[anti-HER2/neu] synthesized using N-ɛ-maleimidocaproic acid hydrazide (EMCH); and (▪) epirubicin-(C3-amide)-[anti-HER2/neu] with epirubicin-(C3-amide)-[anti-EGFR] as a 50/50 epirubicin-equivalent combination synthesized utilizing succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC). Mammary adenocarcinoma SKBr-3 monolayer populations were incubated with covalent epirubicin-immunochemotherapeutics for 72 hours and cytotoxic potency measured as a function of MTT cell vitality stain intensity relative to matched negative reference controls.