Abstract
Rationale
Discounting of delayed and probabilistic reinforcement is linked to increased drug use and pathological gambling. Understanding the neurobiology of discounting is important for designing treatments for these disorders. Glutamate is considered to be involved in addiction-like behaviors; however, the role of ionotropic glutamate receptors (iGluRs) in discounting remains unclear.
Objectives
The current study examined the effects of N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid (AMPA) glutamate receptor blockade on performance in delay and probability discounting tasks.
Methods
Following training in either delay or probability discounting, rats (n = 12, each task) received pretreatments of the NMDA receptor antagonists MK-801 (0, 0.01, 0.03, 0.1, or 0.3 mg/kg, s.c.) or ketamine (0, 1.0, 5.0, or 10.0 mg/kg, i.p.), as well as the AMPA receptor antagonist CNQX (0, 1.0, 3.0, or 5.6 mg/kg, i.p.). Hyperbolic discounting functions were used to estimate sensitivity to delayed/probabilistic reinforcement and sensitivity to reinforcer amount.
Results
An intermediate dose of MK-801 (0.03 mg/kg) decreased sensitivity to both delayed and probabilistic reinforcement. In contrast, ketamine did not affect the rate of discounting in either task but decreased sensitivity to reinforcer amount. CNQX did not alter sensitivity to reinforcer amount or delayed/probabilistic reinforcement.
Conclusions
These results show that blockade of NMDA receptors, but not AMPA receptors, decreases sensitivity to delayed/probabilistic reinforcement (MK-801) and sensitivity to reinforcer amount (ketamine). The differential effects of MK-801 and ketamine demonstrate that sensitivities to delayed/probabilistic reinforcement and reinforcer amount are pharmacologically dissociable.
Keywords: discounting, delayed reinforcement, probabilistic reinforcement, NMDA receptor, AMPA receptor, rat
Introduction
Delay and probability discounting refer to the decrease in subjective value of a reinforcer as a function of the delay to or odds against its delivery, respectively. In general, performance in delay and/or probability discounting tasks is associated with several maladaptive behaviors, including drug use and pathological gambling. For example, drug abusers show greater discounting of delayed monetary rewards relative to non-abusers (Bickel et al. 1999; Coffey et al. 2003; Madden et al. 1997; Mitchell 1999; Vuchinich and Simpson 1998), although other studies show that drug users and non-users do not differ in probability discounting (Andrade and Petry 2002; Mitchell 1999; Ohmura et al. 2005; Reynolds et al. 2007; Yi and Landes 2012). Pathological gamblers show steeper discounting of delayed reinforcement and respond more for large, uncertain reinforcers relative to small, certain reinforcers (Madden et al. 2009; Petry 2001). Among college student participants, delay discounting functions are correlated across reinforcer types (food, sexual stimuli, money), whereas probability discounting functions are not (Holt et al. 2014). Taken together, these results suggest that delay and probability discounting reflect different constructs, likely involving dissociable neurobehavioral mechanisms.
Glutamate is the major excitatory amino acid neurotransmitter involved in learning and memory (see Riedel et al. 2003 for a review) and is considered to play a pivotal role in addiction-like behaviors (Kalivas 2009) and impulse disorders (Lesch et al. 2013; Pattij and Vanderschuren 2008). Because memory processes have been proposed to underlie discounting (Killeen 2011), glutamatergic functioning is a potential mechanism underlying sensitivity to delayed and/or uncertain reinforcement. Indeed, evidence suggests that elevated glutamate levels are associated with increased delay discounting (Schmaal et al. 2012). Blockade of N-methyl-D-aspartate (NMDA) receptors has also been demonstrated to increase delay discounting (Cottone et al. 2013; Floresco et al. 2008). However, it is not known if NMDA antagonists alter delay and probability discounting differentially, nor is it known if other iontropic receptors are involved. Thus, the goals of the current study were to determine if NMDA receptors differentially mediate delay and probability discounting performance and to determine if α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid (AMPA) receptors are involved in discounting.
Methods
Subjects
A total of 48 Sprague Dawley rats were tested in either a delay discounting (n = 24) or probability discounting (n = 24) task. They were acclimated to a colony room held at a constant temperature and handled for 5 days upon arrival. Rats had no prior operant training before the current experiment; however, during adolescence, some rats were treated with amphetamine (1.0 mg/kg; 4 injections) and saline (1.0 ml/kg; 4 injections) on alternating days (n = 12), and some were only treated with saline (8 injections; n = 12). Amphetamine treatment during adolescence did not significantly alter discounting of delayed or probabilistic reinforcement in the current study (data not shown). Rats were individually-housed during the current experiment. Light and dark phases were on a 12:12 h cycle, and all procedures occurred in the light phase. Rats were food restricted (given 10 g/day) during behavioral studies. All procedures were in accordance with the “Guide for the Care and Use of Laboratory Animals” (National Research Council 2011) and were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
Drugs
(+)-MK-801 hydrogen maleate, (±)-ketamine hydrochloride, and 6-cyano-7-nitroquinoxaline-2,3-dione disodium salt hydrate (CNQX; Sigma, St. Louis, MO) were prepared in sterile 0.9% NaCl (saline) and injected in a volume of 1 ml/kg. The doses were calculated based on salt weight.
Apparatus
Operant conditioning chambers (28 × 21 × 21 cm; ENV-008; MED Associates, St. Albans, VT) located inside sound-attenuating chambers (ENV-018M; MED Associates) were used. The front and back walls of the experimental chambers were made of aluminum, while the side walls were made of Plexiglas. There was a recessed food tray (5 × 4.2 cm) located 2 cm above the floor in the bottom-center of the front wall. An infrared photobeam was used to record head entries into the food tray. A 28-V white cue light was located 6 cm above each response lever. A white house light was mounted in the center of the back wall of the chamber. All responses and scheduled consequences were recorded and controlled by a computer interface. A computer controlled the experimental session using Med-IV software.
Procedure
Rats were given 2 days of magazine training, in which sucrose-based 45 mg pellets (F0021 dustless precision pellet, Bio-Serve, Frenchtown, NJ) were non-contingently delivered into the food tray. Following magazine training, rats were given lever press training. Each session began with illumination of the house light. A head entry into the food hopper resulted in presentation of one lever. Levers were presented randomly, with no more than two consecutive presentations of the same lever. A response on either lever resulted in delivery of one sucrose pellet. Pellets were also delivered non-contingently on a random time 100 sec schedule of reinforcement. Following a response on either lever, the house light was extinguished, and the lever was retracted for 5 sec. After 5 sec, the house light was illuminated. Each session lasted 30 min.
After 3 sessions, rats received reward magnitude discrimination training, which consisted of 40 trials. Each trial lasted 40 sec and began with illumination of the house light. A head entry into the food hopper extended one of the levers (randomly presented, with no more than two consecutive presentations of the same lever). A response on one lever resulted in immediate delivery of one pellet, whereas a response on the other lever resulted in immediate delivery of four pellets (the lever associated with the large reward magnitude was counterbalanced across rats). Following a response, the house light was extinguished, and the lever was retracted for the remainder of the trial. If a response was not made within 10 sec, the trial was scored as an omission, and the house light was extinguished for the remainder of the trial. After 7 days of reward magnitude discrimination training, rats were trained in either a delay discounting or probability discounting task.
Discounting sessions consisted of 5 blocks of 9 trials (delay discounting) or 18 trials (probability discounting), and each trial lasted either 60 sec (delay discounting) or 30 sec (probability discounting). The first 4 trials (delay discounting) or 8 trials (probability discounting) in a block were forced-choice trials, in which only one lever was randomly presented (no more than 2 consecutive presentations of the same lever). The remaining trials were free-choice trials, in which both levers were extended. As in reward magnitude discrimination training, a response on one lever always resulted in immediate delivery of one food pellet. A response on the other lever resulted in either delayed or probabilistic delivery of 4 pellets. The delay to or odds against (odds against = [1-probability]/probability) the delivery of the large magnitude reward increased across blocks of trials. The delays to the larger reinforcer were 0, 5, 10, 20, and 50 sec, and the odds against obtaining reinforcement were 0, 1, 3, 7, and 15. Following a response on either lever, the house light was extinguished, and the lever was retracted for the remainder of the trial. If a response was not made within 10 sec, the trial was scored as an omission, and the house light was extinguished for the remainder of the trial.
Following training, rats (n = 12 each task) received various doses of the NMDA receptor antagonist MK-801 (0, 0.01, 0.03, 0.1, or 0.3 mg/kg, s.c.), with each dose administered 15 min prior to the session. The doses and pretreatment time were chosen based on previous work (Almasi-Nasrabadi et al. 2012; Fredriksson and Archer 2002; Wooters et al. 2011). A separate group of rats (n = 12 each task) received various doses of the NMDA receptor antagonist ketamine (0, 1.0, 5.0, or 10.0 mg/kg, i.p.) 15 min prior to the session. The doses and pretreatment time were chosen based on previous work (Cottone et al. 2013; Floresco et al. 2008; Oliver et al. 2009). A subset of rats treated with MK-801 (n = 6 each task) and ketamine (n = 6 each task) received additional training in the discounting tasks before receiving various doses of the AMPA receptor antagonist CNQX (0, 1, 3, or 5.6 mg/kg, i.p.) 20 min prior to the session. The doses and pretreatment time were chosen based on previous work (Bäckström and Hyytiä 2004; Wooters et al. 2011). In each experiment, pretreatments occurred once every 4 days, and dose order was randomized.
Data Analysis
To determine if MK-801 or CNQX altered delay or probability discounting, the hyperbolic discounting function was fit to each individual’s data as defined by the equation V = A/(1+bX), where V is the subjective value of the reinforcer, A is reinforcer amount, b is the rate of discounting, and X represents the delay to or odds against reinforcer delivery (Mazur 1987). This analysis was chosen because sensitivity to delayed/probabilistic reinforcement and sensitivity to reinforcer amount have been proposed to independently influence discounting of a reinforcer (Ho et al. 1999). The median proportion of variance accounted for by the hyperbolic discounting function was .83 for delay discounting and .86 for probability discounting.
Because omissions and A parameter estimates were not normally distributed, these data were analyzed with Friedman tests. Main effects were probed with Wilcoxon signed-ranked post hoc tests. Log-transformed b parameter estimates were analyzed with repeated measures analyses of variance (ANOVA), with treatment as a within-subjects factor. When the assumption of sphericity was violated, degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity. Main effects were probed with Bonferroni post hoc tests. Significance was defined as p < .05 in all cases, except for the use of Wilcoxon signed-ranked post hoc test, in which a Bonferroni adjustment was used to correct for multiple comparisons.
Results
Figure 1 shows the proportion of choices for the delayed/probabilistic reinforcer following each dose of MK-801, ketamine, and CNQX. The proportion of choices for the large delayed/probabilistic reinforcer decreased as function of the delay to or odds against receiving reinforcement, although the highest doses of MK-801 (0.1 and 0.3 mg/kg) flattened the discounting functions in both tasks. The flattening of the discounting function following MK-801 (0.1 and 0.3 mg/kg) can be attributed to a loss in schedule control, as these doses significantly increased omissions in delay (χ2(4) = 41.02, p < .05; Figure 2a) and probability discounting tasks (χ2(4) = 38.51, p < .05; Figure 2b). Because the higher doses (0.1 and 0.3 mg/kg) of MK-801 produced a general suppression in behavior, these doses were excluded from subsequent analyses of parameters b and A. Although ketamine did not increase the number of omissions during the delay discounting task (Figure 2c), ketamine (10.0 mg/kg) significantly increased omissions during the probability discounting task (χ2(3) = 14.20, p < .05; Figure 2d). Similar to ketamine, CNQX did not significantly alter omissions in delay discounting (Figure 2e), but the highest dose (5.6 mg/kg) significantly increased omissions in probability discounting (χ2(3) = 8.48, p < .05 Figure 2f).
Figure 1.
Mean (± SEM) proportion of choices for the large magnitude reinforcer as a function of the delay to or odds against receiving reinforcement in the delay and probability discounting tasks following pretreatments of MK-801 (a and b, respectively; n = 12 each task), ketamine (c and d, respectively; n = 12 each task) and CNQX (e and f, respectively; n = 12 each task).
Figure 2.
Mean (± SEM) omissions during free-choice trials in the delay and probability discounting following pretreatments of MK-801 (a and b, respectively; n = 12 each task), ketamine (c and d, respectively; n = 12 each task), and CNQX (e and f, respectively; n = 12 each task). *p < .05, relative to vehicle. Note the different y-axes for delay and probability discounting tasks.
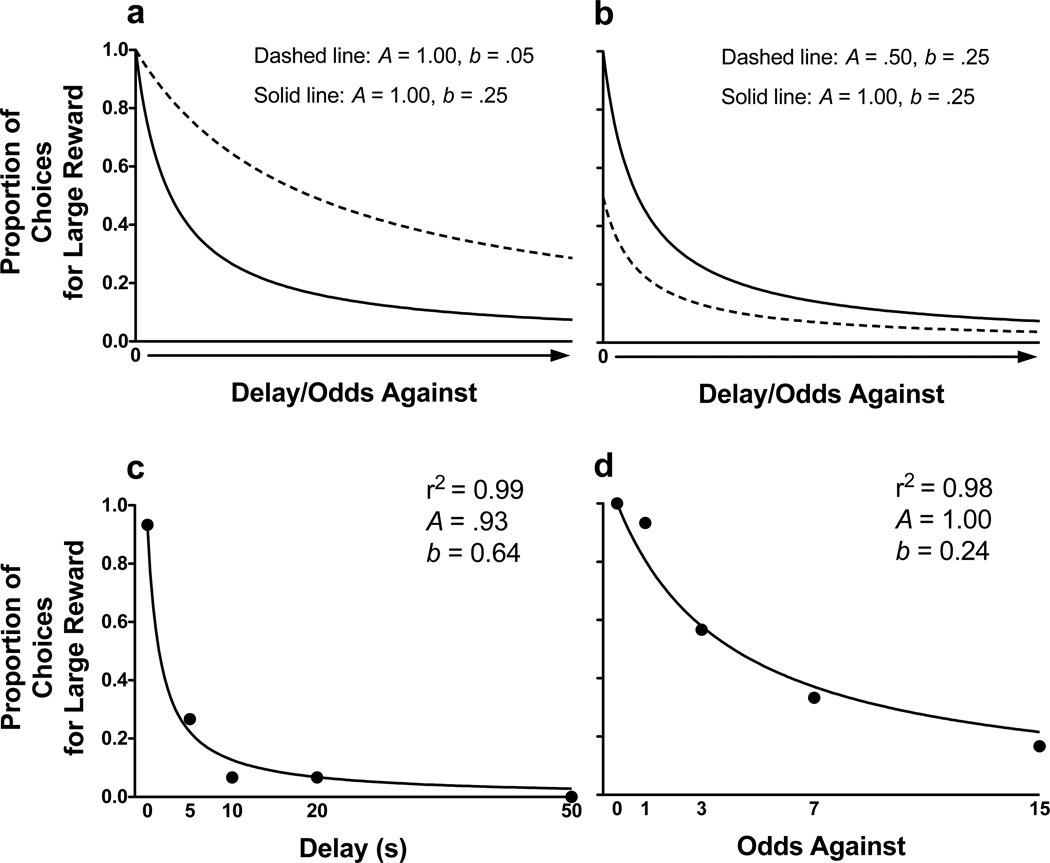
Figure 3a shows two theoretical hyperbolic discounting functions highlighting differential sensitivity to delayed/probabilistic reinforcement (parameter b) with no differences in sensitivity to reinforcer amount (parameter A). Figure 3b shows two theoretical discounting functions highlighting different A parameter estimates with no change in parameter b. Figures 3c and 3d show the hyperbolic discounting function fit to representative subjects performing in delay discounting (Figure 3c) and probability discounting (Figure 3d) in the absence of drug.
Figure 3.
Theoretical hyperbolic discounting curves showing a differential sensitivity to delayed/probabilistic reinforcement discounting (parameter b) and b differential sensitivity to reinforcer magnitude (parameter A). Representative hyperbolic discounting function fit to individual subjects performing in the delay discounting or probability discounting task (c and d, respectively).
Parameters b and A were derived and plotted in Figure 4 and Figure 5 for each drug dose, except for the two highest doses of MK-801 (0.1 and 0.3 mg/kg) for delay and probability discounting. MK-801 (0.03 mg/kg) decreased sensitivity to delayed reinforcement (F(2, 22) = 5.04, p < .05; Figure 4a) and probabilistic reinforcement (F(2, 22) = 9.68, p < .01; Figure 4b). Ketamine and CNQX did not significantly alter sensitivity to delayed/probabilistic reinforcement (Figures 4c-4f). MK-801 increased sensitivity to reinforcer magnitude in the delay discounting task (χ2(2) = 7.64, p < .05; Figure 5a), although post hoc tests revealed no significant differences between doses (p’s > .025, Bonferroni correction; Figure 5a). MK-801 did not alter sensitivity to reinforcer amount in the probability discounting task (Figure 5b). In contrast to MK-801, ketamine significantly decreased sensitivity to reinforcer amount in delay discounting (χ2(3) = 9.81, p < .05; Figure 5c) and probability discounting (χ2(3) = 18.85, p < .05; Figure 5d). Post-hoc tests showed that the highest dose of ketamine (10.0 mg/kg) decreased sensitivity to reinforcer amount in both tasks (p’s < .017, Bonferroni correction). Furthermore, a dose of ketamine (5.0 mg/kg) that did not produce an increase in omissions decreased sensitivity to reinforcer amount in the probability discounting task (p < .017, Bonferroni correction). CNQX did not alter sensitivity to reinforcer magnitude in either task (Figures 5e and 5f).
Figure 4.
Mean (± SEM) parameter estimate b values obtained for delay and probability discounting following pretreatments of MK-801 (a and b, respectively; n = 12 each task), ketamine (c and d, respectively; n = 12 each task), and CNQX (e and f, respectively; n = 12 each task). *p < .05, relative to vehicle. Note that log transformed b values were used for data analyses.
Figure 5.
Mean (± SEM) parameter estimate A values obtained for delay and probability discounting following pretreatments of MK-801 (a and b, respectively; n = 12 each task), ketamine (c and d, respectively; n = 12 each task), and CNQX (e and f, respectively; n = 12 each task). *p < .05, relative to vehicle. Note the different y-axis for ketamine relative to MK-801 and CNQX.
Discussion
There were three important findings to the current study. First, NMDA receptor blockade with MK-801 dose-dependently decreased sensitivity to both delayed and probabilistic reinforcement. The decrease in sensitivity in both tasks occurred at a dose of MK-801 (0.03 mg/kg) that did not alter omissions, suggesting a specific effect on choice behavior. Second, NMDA receptor blockade with ketamine decreased sensitivity to reinforcer amount without altering sensitivity to delayed/probabilistic reinforcement. Similar to the finding with MK-801 (0.03 mg/kg), the decrease in sensitivity to reinforcer amount occurred at a dose of ketamine (5.0 mg/kg in probability discounting; 10.0 mg/kg in delay discounting) that did not alter omissions. Third, AMPA receptor blockade with CNQX did not affect sensitivity to delayed/probabilistic reinforcement or sensitivity to reinforcer amount. Together, these results demonstrate that glutamate mediates delay and probability discounting via NMDA receptors, and the NMDA receptor antagonists MK-801 and ketamine produce differential effects on sensitivity to delayed/probabilistic reinforcement (parameter b) and sensitivity to reinforcer amount (parameter A).
The finding that MK-801 decreased delay discounting contrasts with previous findings that administration of ketamine and the NMDA receptor antagonist memantine increase delay discounting (Cottone et al. 2013; Floresco et al. 2008). One important consideration is that MK-801, ketamine, and memantine differentially interact with NMDA receptor channels. Specifically, MK-801 has higher potency relative to ketamine and memantine (Parsons et al. 1995); however, ketamine and memantine bind with the channel faster (Parsons et al. 1995) and show faster unblocking kinetics relative to MK-801 (see Danysz et al. 1997 for a review). Furthermore, ketamine and memantine reduce NMDA receptor-mediated excitatory postsynaptic potentials in a voltage-dependent manner, whereas the effects of MK-801 on postsynaptic potentials appear to be less voltage-dependent (Frankiewicz et al. 1996; Halliwell et al. 1989).
Another important consideration for comparing results across studies is that MK-801, ketamine, and memantine show differential selectivity for non-NMDA receptors. For example, MK-801 inhibits nicotinic acetylcholine receptors (Amador and Dani 1991); however, blockade of nicotinic receptors does not alter discounting of delayed or probabilistic reinforcement (Mendez et al. 2012). As for dopamine activity, previous work has shown that acute administration of MK-801 (0.03 and 0.1 mg/kg) increases dopamine levels in prefrontal cortex (Tsukada et al. 2005). Interestingly, drugs that increase dopamine levels typically decrease sensitivity to delayed and uncertain reinforcement (e.g., Koffarnus et al. 2011; St Onge and Floresco 2009). Thus, the decrease in discounting observed following MK-801 administration might be explained from its interaction with the prefrontal dopamine system. In contrast to MK-801, ketamine acts at sigma receptors (Robson et al. 2012) and opioid receptors (Gupta et al. 2011). Ketamine also acts as an antagonist at 5-HT2 receptors (Kapur and Seeman 2002), and memantine blocks 5-HT3 receptors (Rammes et al. 2001), although antagonism of 5-HT receptors typically does not increase the rate of discounting (Hadamitzky et al. 2009; Liu et al. 2004; Talpos et al. 2006). Future studies will need to further characterize the specific role that NMDA receptors play in delay and probability discounting performance.
While MK-801 produced a clear upward shift in the discounting function, it should be noted that MK-801 increases food intake in rats (Burns and Ritter 1997; Ninan and Kulkarni 1998). Because MK-801 increases food consumption, this could motivate animals to earn more food during the discounting procedure. Indeed, an increase in the A parameter was observed following administration in the delay discounting task. However, altered hunger motivation does not provide a cogent explanation of the results because sensitivity to reinforcer amount was not altered in the probability discounting task. Furthermore, b and A independently influence discounting performance (see Ho et al. 1999). If the alteration in discounting following MK-801 administration was due to a change in hunger motivation, one would expect to observe a change in A only. Another argument against a role for altered hunger motivation is that ketamine, which has been shown to increase sweet food consumption (Garcia et al. 2009), caused a decrease in sensitivity to reinforcer amount in both the delay and probability discounting task.
The ketamine-induced specific decrease in reinforcer amount sensitivity observed in the current study seems to contrast with the ketamine-induced increase in delay discounting reported previously (Floresco et al. 2008; Cottone et al. 2013). However, there are at least two procedural differences that may account for the discrepant findings observed across studies. First, the number of forced/free choice trials differed across studies; the current study used four forced-choice trials and five free-choice trials, whereas Floresco et al. (2008) used two forced-choice and 10 free-choice trials. We chose to use fewer trials in our procedure to limit the maximum number of pellets a rat could earn in a session (150 pellets for an individual rat during a single session), and we wanted to ensure that the number of pellets earned in the delay discounting procedure was similar to the pellets earned in the probability discounting procedure (between 90–150 pellets in each task). It is unlikely that the number of trials affected discounting performance because animals performing the probability discounting task received twice as many trials (eight forced-choice and 10 free-choice) and showed similar effects in discounting performance following MK-801 and ketamine administration. Second, another important consideration is the analyses used across the two studies. Floresco et al. (2008) used an ANOVA to analyze the percentage of responses for the large reinforcer, whereas the current study used the hyperbolic discounting function to generate parameter estimates of b (sensitivity to delayed/probabilistic reinforcement) and A (sensitivity to reinforcer amount). Importantly, ketamine administration appeared to produce a parallel negative shift in the discounting curve as in the Floresco et al. (2008) study, suggesting that sensitivity to reinforcer magnitude, but not the rate of discounting, was altered. To add further support of this interpretation, the current results show that ketamine administration had no effect on sensitivity to delayed/probabilistic reinforcement, but decreased sensitivity to reinforcer amount (parameter A). Collectively, the current results coincide with those of Floresco et al. (2008), and indicate that ketamine’s effects are specific to the reward amount discrimination within both delay and probability discounting.
Delay and probability discounting may measure similar or distinct constructs of impulsivity. It has been proposed that delay and probability discounting reflect a similar underlying process (Myerson and Green 1995; Rachlin et al. 1991). In support of this proposal, the same mathematical functions (e.g., hyperbolic) can be used to model delay and probability discounting (Rachlin et al. 1991). Also, administration of amphetamine produces similar shifts in delay (e.g., van Gaalen et al. 2006; Winstanley et al. 2003) and probability discounting (e.g., Floresco and Whelan 2009; St Onge and Floresco 2009). However, other evidence suggests that delay and probability discounting involve distinct processes, with delay and probability discounting reflecting impulsive choice and risky decision making, respectively (Ainslie 1975; Kahneman and Tversky 1979). In support of this latter view, manipulating reinforcer magnitude differentially alters discounting of delayed and uncertain reinforcement (Green et al. 1999), and forebrain depletion of serotonin increases sensitivity to delayed reinforcement without altering sensitivity to probabilistic reinforcement (Mobini et al. 2000). If this interpretation is accurate, the current results suggest that NMDA receptors differentially mediate impulsivity, with MK-801 decreasing impulsive choice but increasing risky decision making. This distinction is important to make in order to effectively treat individuals diagnosed with different psychiatric disorders characterized by impulsive or risky decision making. In this case, prescribing an individual with a drug similar to MK-801 may be beneficial for someone high in impulsive choice; however, the same medication may be ineffective in someone with pathological gambling.
In conclusion, results from the current study show that NMDA receptor antagonists differentially mediate sensitivity to reinforcer amount and sensitivity to delayed/probabilistic reinforcement. Blockade of NMDA receptors with the non-competitive antagonist MK-801 decreased sensitivity to delayed and uncertain reinforcement to a similar extent, whereas antagonism of NMDA receptors with ketamine decreased sensitivity to reinforcer amount without altering delay/probability discounting. Understanding the precise role of glutamate systems in delay and probability discounting might be beneficial in developing treatments for disorders such as drug abuse or pathological gambling.
Acknowledgements
The authors would like to thank Emily Denehy and Travis McCuddy for technical assistance.
The current study was supported by NIH grants P50 DA05312, R01 DA12964, K99 DA 033373, and T32 DA016176.
Footnotes
The authors declare no conflict of interest.
References
- Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82:463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Almasi-Nasrabadi M, Javadi-Paydar M, Mahdavian S, Babaei R, Sharifian M, Norouzi A, Dehpour AR. Involvement of NMDA receptors in the beneficial effects of pioglitazone on scopolamine-induced memory impairment in mice. Behav Brain Res. 2012;231:138–145. doi: 10.1016/j.bbr.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Amador M, Dani JA. MK-801 inhibition of nicotinic acetylcholine receptor channels. Synapse. 1991;7:207–215. doi: 10.1002/syn.890070305. [DOI] [PubMed] [Google Scholar]
- Andrade LF, Petry NM. Delay and probability discounting in pathological gamblers with and without a history of substance use problems. Psychopharmacology. 2012;219:491–499. doi: 10.1007/s00213-011-2508-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckström P, Hyytiä P. Ionotropic glutamate receptor antagonists modulate cue-induced reinstatement of ethanol-seeking behavior. Alcohol Clin Exp Res. 2004;28:558–565. doi: 10.1097/01.alc.0000122101.13164.21. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Burns GA, Ritter RC. The non-competitive NMDA antagonist MK-801 increases food intake in rats. Pharmacol Biochem Behav. 1997;56:145–149. doi: 10.1016/S0091-3057(96)00171-2. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacology. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Cottone P, Lemolo A, Narayan AR, Kwak J, Momaney D, Sabino V. The uncompetitive NMDA receptor antagonists ketamine and memantine preferentially increase the choice for a small, immediate reward in low-impulsive rats. Psychopharmacology. 2013;226:127–138. doi: 10.1007/s00213-012-2898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danysz W, Parson CG, Kornhuber J, Schmidt WJ, Quack G. Aminoadamantanes as NMDA receptor antagonists and antiparkinsonian agents - preclinical studies. Neurosci Biobehav Rev. 1997;21:455–468. doi: 10.1016/s0149-7634(96)00037-1. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MT, Ghods-Sharifi S. Dopaminergic and glutamateric regulation of effort- and delay-based decision making. Neuropsychopharmacology. 2008;33:1966–1979. doi: 10.1038/sj.npp.1301565. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Whelan JM. Perturbations in different forms of cost/benefit decision making induced by repeated amphetamine exposure. Psychopharmacology. 2009;205:189–201. doi: 10.1007/s00213-009-1529-0. [DOI] [PubMed] [Google Scholar]
- Frankiewicz T, Potier B, Bashir ZI, Collingridge GL, Parsons CG. Effects of memantine and MK-801 on NMDA-induced currents in cultured neurons and on synaptic transmission and LTP in area CA1 of rat hippocampal slices. Br J Pharmacol. 1996;117:689–697. doi: 10.1111/j.1476-5381.1996.tb15245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson A, Archer T. Functional alteration by NMDA antagonist: effects of L-Dopa, neuroleptics and postnatal administration. Amino Acids. 2002;23:111–132. doi: 10.1007/s00726-001-0117-3. [DOI] [PubMed] [Google Scholar]
- Garcia LS, Comim CM, Valvassori SS, Réus GZ, Stertz L, Kapczinski F, Gavioli EC, Quevedo J. Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:450–455. doi: 10.1016/j.pnpbp.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J, Ostaszewski P. Amount of reward has opposite effects on the discounting of delayed and probabilistic outcomes. J Exp Anal Psychol Learn Mem Cogn. 1999;25:418–427. doi: 10.1037//0278-7393.25.2.418. [DOI] [PubMed] [Google Scholar]
- Gupta A, Devi LA, Gomes I. Potentiation of mu-opioid receptor-mediated signaling by ketamine. J Neurochem. 2011;119:294–302. doi: 10.1111/j.1471-4159.2011.07361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadamitzky M, Feja M, Becker T, Koch M. Effects of acute systemic administration of serotonin2A/C receptor ligands in a delay-based decision-making task in rats. Behav Pharmacol. 2009;20:415–423. doi: 10.1097/FBP.0b013e3283305e11. [DOI] [PubMed] [Google Scholar]
- Halliwell RF, Peters JA, Lambert JJ. The mechanism of action and pharmacological specificity of the anticonvulsant NMDA antagonist MK-801: a voltage clamp study on neuronal cells in culture. Br J Pharmacol. 1989;96:480–494. doi: 10.1111/j.1476-5381.1989.tb11841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M-Y, Mobini S, Chiang T-J, Bradshaw CM, Szabadi E. Theory and method in the quantitative analysis of “impulsive choice” behaviour: implications for psychopharmacology. Psychopharmacology. 1999;146:362–372. doi: 10.1007/pl00005482. [DOI] [PubMed] [Google Scholar]
- Holt DD, Newquist MH, Smits RR, Tiry AM. Discounting of food, sex, and money. Psycho Bull Rev. 2014;21:794–802. doi: 10.3758/s13423-013-0557-2. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47:263–292. [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kapur S, Seeman P. NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D2 and serotonin 5-HT2 receptors - implications for models of schizophrenia. Mol Psychiatry. 2002;7:837–844. doi: 10.1038/sj.mp.4001093. [DOI] [PubMed] [Google Scholar]
- Killeen PR. Models of trace decay, eligibility for reinforcement, and delay of reinforcement gradients, from exponential to hyperboloid. Behav Processes. 2011;87:57–63. doi: 10.1016/j.beproc.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Newman AH, Grundt P, Rice KC, Woods JH. Effects of selective dopaminergic compounds on a delay-discounting task. Behav Pharmacol. 2011;22:300–311. doi: 10.1097/FBP.0b013e3283473bcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Merker S, Reif A, Novak M. Dances with black widow spiders: dysregulation of glutamate signalling enters center stage in ADHD. Eur J Neuropharmacol. 2013;23:479–491. doi: 10.1016/j.euroneuro.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Liu YP, Wilkinson LS, Robbins TW. Effects of acute and chronic buspirone on impulsive choice and efflux of 5-HT and dopamine in hippocampus, nucleus accumbens and prefrontal cortex. Psychopharmacology. 2004;173:175–185. doi: 10.1007/s00213-003-1726-1. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharmacology. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Johnson PS. Pathological gamblers discount probabilistic rewards less steeply than matched controls. Exp Clin Psychopharmacology. 2009;17:283–290. doi: 10.1037/a0016806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. The effect of delay and of intervening events on reinforcement value. Erlbaum, Hillsdale: 1987. pp. 53–73. [Google Scholar]
- Mendez IA, Gilbert RJ, Bizon JL, Setlow B. Effects of acute administration of nicotine and muscarinic cholinergic agonists and antagonists on performance in different cost-benefit decision making tasks in rats. Psychopharmacology. 2012;224:489–499. doi: 10.1007/s00213-012-2777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Mobini S, Chiang T-J, Ho M-Y, Bradshaw CM, Szabadi E. Effects of central 5-hydroxytryptamine depletion on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2000;152:390–397. doi: 10.1007/s002130000542. [DOI] [PubMed] [Google Scholar]
- Myerson J, Green L. Discounting of delayed rewards: models of individual choice. J Exp Anal Behav. 1995;64:263–276. doi: 10.1901/jeab.1995.64-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. 8th edition. Washington: National Academies Press; 2011. [PubMed] [Google Scholar]
- Ninan I, Kulkarni SK. Dopamine receptor sensitive effect of dizocilpine on feeding behaviour. Brain Res. 1998;812:157–163. doi: 10.1016/s0006-8993(98)00990-1. [DOI] [PubMed] [Google Scholar]
- Ohmura Y, Takahashi T, Kitamura N. Discounting delayed and probabilistic monetary gains and losses by smokers of cigarettes. Psychopharmacology. 2005;182:508–515. doi: 10.1007/s00213-005-0110-8. [DOI] [PubMed] [Google Scholar]
- Oliver YP, Ripley TL, Stephens DN. Ethanol effects on impulsivity in two mouse strains: similarities to diazepam and ketamine. Psychopharmacology. 2009;204:679–692. doi: 10.1007/s00213-009-1500-0. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Quack G, Bresink I, Baran L, Przegalinski E, Kostowski W, Krzascik P, Hartman S, Danysz W. Comparison of the potency, kinetics and voltage-dependency of a series of uncompetitive NMDA receptor antagonists in vitro with anticonvulsive and motor impairment activity in vivo . Neuropharmacol. 1995;34:1239–1258. doi: 10.1016/0028-3908(95)00092-k. [DOI] [PubMed] [Google Scholar]
- Pattij T, Vanderschuren LJ. The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci. 2008;29:192–199. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Petry NM. Pathological gamblers, with and without substance use disorders, discount delayed rewards at high rates. J Abnorm Psychol. 2001;110:482–487. doi: 10.1037//0021-843x.110.3.482. [DOI] [PubMed] [Google Scholar]
- Rachlin H, Raineri A, Cross D. Subjective probability and delay. J Exp Anal Behav. 1991;55:233–244. doi: 10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammes G, Rupprecht R, Ferrari U, Zieglgänsberger W, Parsons CG. The N-methyl-D-aspartate receptor channel blockers memantine, MRZ 2/579 and other amino-alkyl-cyclohexanes antagonize 5-HT(3) receptor currents in cultured HEK-293 and N1E-115 cell systems in a non-competitive manner. Neurosci Lett. 2001;306:81–84. doi: 10.1016/s0304-3940(01)01872-9. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Patak M, Shroff P, Penfold RB, Melanko S, Duhig AM. Laboratory and self-report assessments of impulsive behavior in adolescent daily smokers and nonsmokers. Exp Clin Psychopharmacology. 2007;15:264–271. doi: 10.1037/1064-1297.15.3.264. [DOI] [PubMed] [Google Scholar]
- Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav Brain Res. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Robson MJ, Elliott M, Seminerio MJ, Matsumoto RR. Evaluation of sigma (σ) receptors in the antidepressant-like effectsof ketamine in vitro and in vivo. Eur Neuropsychopharmacol. 2012;22:308–317. doi: 10.1016/j.euroneuro.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Goudriaan AE, van der Meer J, van den Brink W, Veltman DJ. The association between cingulate cortex glutamate concentration and delay discounting is mediated by resting state functional connectivity. Brain Behav. 2012;2:553–562. doi: 10.1002/brb3.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology. 2009;34:681–697. doi: 10.1038/npp.2008.121. [DOI] [PubMed] [Google Scholar]
- Talpos JC, Wilkinson LS, Robbins TW. A comparison of multiple 5-HT receptors in two tasks measuring impulsivity. J Psychopharmacology. 2006;20:47–58. doi: 10.1177/0269881105056639. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Nishiyama S, Fukumoto D, Sato K, Kakiuchi T, Domino E. Chronic NMDA antagonism impairs working memory, decreases extracellular dopamine, and increases D1 receptor binding in prefrontal cortex of conscious monkeys. Neuropharmacology. 2005;30:1861–1869. doi: 10.1038/sj.npp.1300732. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, van Koten R, Schoffelmeer ANM, Vanderschuren JMJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Exp Clin Psychopharmacology. 1998;6:292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DEH, Robbins TW. Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacology. 2003;170:320–331. doi: 10.1007/s00213-003-1546-3. [DOI] [PubMed] [Google Scholar]
- Wooters TE, Dwoskin LP, Bardo MT. Discriminative stimulus effects of NMDA, AMPA, and mGluR5 glutamate receptor ligands in methamphetamine-trained rats. Behav Pharmacol. 2011;22:516–524. doi: 10.1097/FBP.0b013e328349aafa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R, Landes RD. Temporal and probability discounting by cigarette smokers following acute smoking abstinence. Nicotine Tob Res. 2012;14:547–558. doi: 10.1093/ntr/ntr252. [DOI] [PMC free article] [PubMed] [Google Scholar]