ABSTRACT
Three-dimensional computed tomography (3D-CT) enables in vivo volumetry of total lung volume (TLV) and emphysematous low-attenuation volume (LAV) in patients with chronic obstructive pulmonary disease (COPD). We retrospectively investigated the correlation between preoperative 3D-CT volumetry and postoperative complications in lung cancer patients. We searched our institution’s surgical records from December 2006 to December 2009 and selected patients who had undergone pulmonary lobectomy for primary lung cancer. From 3D-CT data, TLV and LAV <–950 HU of thresholds were retrospectively measured. The LAV% was calculated as follows: LAV% = LAV/TLV*100. The associations between the seven independent variables (LAV%, age, gender, body mass index, smoking history, forced expiratory volume in 1 second as percent forced vital capacity [FEV1%], and resected lobe) and the two outcomes (postoperative complications and prolonged postoperative stay [PPS]) were compared using logistic regression analysis. A total of 309 patients (222 males, 87 females; mean age, 67 years; range, 40–87 years) were evaluated. On multivariate analysis, age and LAV% were significantly correlated with postoperative complications (p = 0.006 and p = 0.006, respectively), and LAV% was significantly correlated with PPS (p = 0.031). LAV% measured using 3D-CT is more sensitive for predicting complications after lobectomy for lung cancer than FEV1%.
Key Words: Chronic obstructive pulmonary disease, Lung cancer, Thoracic surgery, Postoperative complication, Computed tomography
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) often occurs in lung cancer patients due to their smoking history.1) Surgery provides the best chance of prolonged survival for early-stage non-small cell lung cancer. However, postoperative pulmonary complications are among the most common sources of morbidity in patients undergoing major surgery.2) Notably, COPD is the main predictor of perioperative mortality and respiratory morbidity.3,4) Therefore, preoperative patient evaluation for COPD should be performed accurately.
Pulmonary function test (PFT) using a spirometer remains the standard screening test performed before pulmonary resection. However, PFT has a limited role and its results should not be the basis for denying surgery if the surgical indication is compelling, because the severity of COPD based on forced expiratory volume in 1 second (FEV1) by spirometry is imperfectly associated with the presence of symptoms in the individual patient.5)
Computed tomography (CT) is excellent for demonstrating pulmonary emphysema as a low-attenuation area. In addition, development of three-dimensional (3D) CT and computer-aided diagnosis (CAD) enables in vivo 3D volumetry of total lung volume and emphysematous lung volume. Several studies have shown that 3D-CT volumetry can accurately and objectively evaluate the severity of COPD.6-10) Ueda et al. reported that determination of the area of emphysema by quantitative CT is useful in predicting early postoperative oxygenation capacity.11) Therefore, we assume that preoperative 3D-CT volumetry of emphysematous lungs may precisely predict postoperative complications of lung cancer. Accordingly, in the present study we retrospectively investigated the correlation between preoperative 3D-CT volumetry and postoperative complications in lung cancer patients.
MATERIALS AND METHODS
Patient selection
We searched our institution’s surgical records from December 2006 to December 2009 and selected patients who had undergone a pulmonary lobectomy for primary lung cancer in our institution. We then obtained clinical records and preoperative CT images for these patients. For all selected cases, we recorded age, gender, body mass index (BMI), smoking history, forced expiratory volume in 1 second as percent forced vital capacity (FEV1%) measured by spirometry, resected lobe, postoperative complications, and postoperative duration of hospital stay.
Postoperative complications and prolonged postoperative stays
In this study, postoperative complications and prolonged postoperative stays (PPS) were defined as follows based on the definitions used in previous studies.12, 13) Postoperative pulmonary complications included: (i) prolonged oxygen treatment (POT) (the need for oxygen therapy for >2 days or the restart of oxygen therapy); (ii) pneumonia (radiological evidence without bacteriological confirmation was reported as "pneumonia suspected;" radiological evidence including atelectasis and documentation of pathological organism by Gram stain or culture was reported as "pneumonia confirmed"); (iii) prolonged ventilation (PV) (unexpected extubation failure at the end of surgery or postoperative ventilator dependence for >48 hours); (iv) reintubation due to respiratory failure; and (v) prolonged air leakage (bronchial fistula or/and pulmonary fistula). All postoperative pulmonary complications with more than the mild (grade1) described by Common Terminology Criteria for Adverse Events (version 4.0) were detected. Cardiac complications included myocardial infarction, supraventricular arrhythmias, and ventricular arrhythmias, for all of which treatment was needed. Combined cardiopulmonary complications included POT, pneumonia, PV, reintubation due to respiratory failure, prolonged air leakage, and supraventricular arrhythmias. When one patient had some postoperative pulmonary and cardiac complications, we counted one combined cardiopulmonary complication. We defined non-COPD patients as FEV1%≥70%. Mean postoperative stays in non-COPD patients was 11days, therefore, a PPS was defined as a hospital stay of ≥12 days based on the previous study of Matsuo et al.13)
CT scan
All preoperative CT examinations were performed using a 64-detector row scanner (Aquilion 64; Toshiba Medical Systems Corp., Tokyo, Japan). All scans were obtained from the lung apex to the diaphragm, during a breath-hold at deep inspiration, using the following parameters: x-ray tube voltage, 120 kVp; automatic tube-current maximum, 225 mAs; gantry rotation speed, 0.5 sec; and beam collimation, 64×0.5mm. CT images were reconstructed from 5-mm slices with intervals of 5 mm, using a standard algorithm. No intravenous contrast media was administered.
3D-CT volumetry
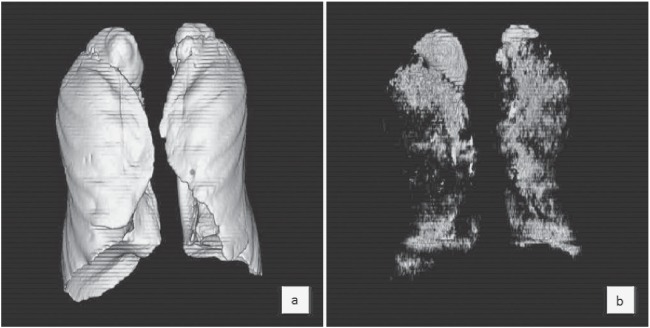
All CT data for each patient were transferred to a computer workstation (ZioStation; Ziosoft, Osaka, Japan), and 2 radiologists (with 18 and 3 years of experience in interpreting thoracic CT) reconstructed 3D models (Fig. 1). Threshold limits of –400 to –1,024 HU were automatically applied to exclude soft tissue surrounding the lung and large vessels within the lung. The 3D model was viewed as a volume-rendering display at multiple angles to ensure that the model was valid. The trachea, main-stem bronchi, and lobar to segmental bronchus were semi-automatically and selectively removed from the 3D model of the whole lung.6, 7) First, the volume of voxels on these 3D images was calculated as total lung volume (TLV). Second, the volume of voxels with attenuation values <–950 HU of thresholds was measured as low-attenuation volume (LAV). Finally, the percentage of LAV per TLV (LAV%) was calculated as follows: LAV% = LAV/TLV*100.
Fig. 1.
Procedure for 3D-CT volumetry. (a) The volume of a three-dimensional image was calculated as total lung volume (TLV). (b) Volumes < –950 HU were measured as low-attenuation volume (LAV). In this case, the the percentage of LAV (LAV%) was 8.5%.
Statistical analysis
The associations between the seven independent variables (LAV%, age, gender, BMI, smoking history, FEV1%, and resected lobe) and the two outcomes (postoperative complications and PPS) were compared using univariate and multivariate logistic regression analysis. For this analysis, patients were assigned to two groups based on the Brinkman index (BI) of 200 for smoking history. Next, we determined a cut-off level that would indicate postoperative complications and PPS for LAV% using receiver operating characteristic (ROC) curve analysis and Youden’s index. Excel 2007 (Microsoft Corp., Redmond, WA) and SPSS, version 21.0 (IBM Corp., Armonk, NY) were used to conduct statistical analyses. A p-value of <0.05 was considered significant.
RESULTS
Study cohort
A total of 309 patients (222 males and 87 females; mean age, 67 years; range, 40–87 years) were enrolled. Other patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| Mean ± SD | Range | ||
|---|---|---|---|
| Age (years) | 67 ± 8 | 40 – 87 | |
| BMI (kg/m2) | 22.2 ± 3.5 | 13.4 – 38.3 | |
| 3D-CT volumetry | |||
| TLV (mL) | 4494 ± 1092 | 1507 – 7668 | |
| LAV (mL) | 267 ± 509 | 0 – 3825 | |
| LAV% (%) | 5.0 ± 8.4 | 0 – 51.6 | |
| Spirometry | |||
| %VC (%) | 108.0 ± 17.5 | 62.6 – 199.0 | |
| FEV1% (%) | 71.0 ± 10.5 | 25.6 – 98.0 | |
| %DLco/VA (%) | 95.9 ± 27.9 | 23.7 – 181.5 | |
BMI, body mass index; 3D-CT, three-dimensional computed tomography; TLV, total lung volume; LAV, low-attenuation volume; VC, vital capacity; FEV1, forced expiratory volume in 1 second; DLco, carbon monoxide diffusing capacity; VA, alveolar ventilation
Two hundred forty patients (77.7%) had a smoking history and one hundred thirty-three patients (43.0%) demonstrated obstructive abnormality (FEV1% <70%). The global initiative for chronic obstructive lung disease (GOLD) stage distribution was as follows: stage I (n=98), stage II (n=32), and stage III (n=3). The mean ± standard deviation (SD) LAV% of non-COPD, GOLD stage I, II, and III group were 2.5 ± 4.9%, 5.4 ± 6.6%, 14.4 ± 14.4%, and 33.6 ± 16.7%, respectively. Resected lobes included the right upper lobe (n=105), right middle lobe (n=18), right lower lobe (n=51), right middle and lower lobe (n=9), left upper lobe (n=89), and left lower lobe (n=37). Pathological analysis of postoperative specimens confirmed 197 adenocarcinomas, 71 squamous cell carcinomas, 15 adenosquamous carcinomas, and 26 other. Postoperative complications were observed in 120 patients (38.8%); these data are shown in Table 2. The mean ± standard deviation (SD) postoperative duration of hospitalization was 12.8 ± 17.4 days (range, 5–291 days), and 108 patients (35.0%) stayed for ≥12 days.
Table 2.
Postoperative complications
| n | |
|---|---|
| Total | 120 |
| Prolonged oxygen treatment | 84 |
| Cardiac complication | 43 |
| Pneumonia | 19 |
| Prolonged air leakage | 19 |
| Prolonged ventilation / Reintubation | 10 |
Association between independent variables and postoperative complications
Table 3 shows the association between independent variables and postoperative complications using univariate and multivariate logistic analysis. On univariate analysis, gender, age, smoking history, LAV%, and FEV1% were significantly correlated with postoperative complications (p = 0.001, p = 0.010, p = 0.003, p < 0.001, and p = 0.004, respectively), while BMI and resected lobe were not correlated with complications (p = 0.122 and p = 0.665, respectively). On multivariate analysis, only age and LAV% were significantly correlated with postoperative complications (p = 0.006 and p = 0.006, respectively).
Table 3.
Correlation between independent variables and postoperative complications
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Independent variables | OR | 95% CI | p-value | OR | 95% CI | p-value |
| Gender (Male vs. Female) |
2.530 | 1.448 – 4.419 | 0.001 | 1.711 | 0.839 – 3.489 | 0.139 |
| Age | 1.038 | 1.009 – 1.067 | 0.010 | 1.043 | 1.012 – 1.075 | 0.006 |
| BMI | 0.948 | 0.887 – 1.014 | 0.122 | 0.984 | 0.910 – 1.065 | 0.691 |
| Smoking history (BI≥200 vs. BI<200) |
2.533 | 1.370 – 4.684 | 0.003 | 1.596 | 0.738 – 3.453 | 0.235 |
| Resected lobe (Others vs. Right upper lobectomy) |
0.968 | 0.836 – 1.121 | 0.665 | 0.988 | 0.844 – 1.156 | 0.875 |
| FEV1% | 0.967 | 0.945 – 0.989 | <0.004 | 1.004 | 0.973 – 1.029 | 0.980 |
| LAV% | 1.073 | 1.038 – 1.110 | <0.001 | 1.056 | 1.016 – 1.098 | 0.006 |
OR, odds ratio; CI, confidence interval; BMI, body mass index; BI, brinkman index; FEV1, forced expiratory volume in 1 second; LAV, low-attenuation volume
Association between independent variables and PPS
Table 4 shows the association between independent variables and postoperative complications using univariate and multivariate logistic analysis. On univariate analysis, gender, smoking history, LAV%, and FEV1% were significantly correlated with PPS (p = 0.0027, p = 0.005, p < 0.001, and p = 0.007, respectively), while age, BMI, and resected lobe were not (p = 0.330, p = 0.326, and p = 0.547, respectively). On multivariate analysis, only LAV% was significantly correlated with PPS (p = 0.031).
Table 4.
Correlation between independent variables and PPS
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Independent variables | OR | 95% CI | p-value | OR | 95% CI | p-value |
| Gender (Male vs. Female) |
1.868 | 1.074 – 3.250 | 0.027 | 1.076 | 0.535 – 2.166 | 0.837 |
| Age | 1.014 | 0.986 – 1.042 | 0.330 | 1.016 | 0.987 – 1.045 | 0.294 |
| BMI | 0.966 | 0.903 – 1.035 | 0.326 | 0.134 | 0.003 – 6.177 | 0.903 |
| Smoking history (BI≥200 vs. BI<200) |
2.529 | 1.332 – 4.804 | 0.005 | 2.023 | 0.920 – 4.451 | 0.080 |
| Resected lobe (Others vs. Right upper lobectomy) |
1.047 | 0.901 – 1.126 | 0.547 | 1.070 | 0.914 – 1.252 | 0.399 |
| FEV1% | 0.969 | 0.947 – 0.991 | 0.007 | 0.993 | 0.965 – 1.021 | 0.617 |
| LAV% | 1.055 | 1.024 – 1.088 | <0.001 | 1.042 | 1.004 – 1.081 | 0.031 |
OR, odds ratio; CI, confidence interval; BMI, body mass index; BI, brinkman index; FEV1, forced expiratory volume in 1 second; LAV, low-attenuation volume
Determination of suitable cut-off values for predicting postoperative outcomes
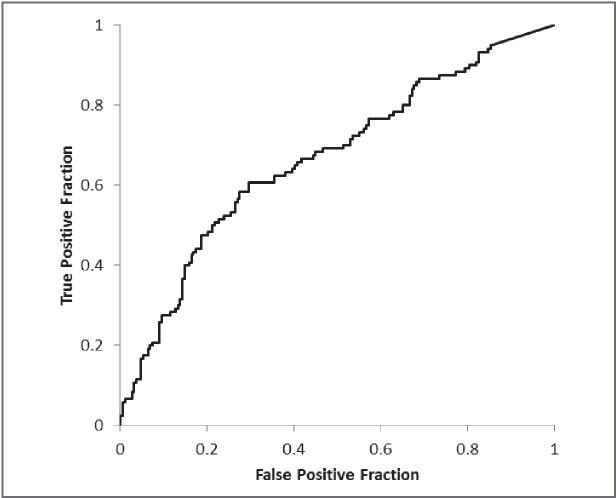
In the analysis of postoperative complications the results of the ROC analysis for LAV% showed an area under the curve (AUC) of 0.668 (Fig. 2). A suitable cut-off value for determining complications was estimated to be 2.2%. This value yielded a sensitivity and specificity for postoperative complications of 60.8% and 70.4%, respectively. Of 129 patients who demonstrated an LAV% >2.2% on 3D-CT, 44 patients demonstrated normal FEV1% values (≥70%) on spirometry.
Fig. 2.
The ROC curve for the percentage of low-attenuation volume (LAV%) in the postoperative complications analysis. The area under the curve (AUC) of 0.668. A suitable cut-off value for determining complications was estimated to be 2.2%.
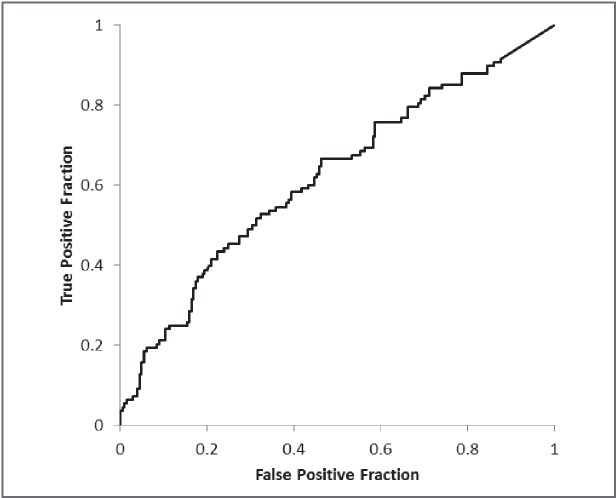
In the PPS analysis, the results of the ROC analysis for LAV% showed an AUC of 0.621 (Fig. 3). A suitable cut-off value for determining PPS was estimated to be 4.6%. This value yielded a sensitivity and specificity for PPS of 43.5% and 77.6%, respectively. Of 90 patients who demonstrated an LAV% >4.6% on 3D-CT, 29 patients demonstrated normal FEV1% values on spirometry.
Fig. 3.
The ROC curve for the percentage of low-attenuation volume (LAV%) in the prolonged postoperative stay (PPS) analysis. The area under the curve (AUC) of 0.621. A suitable cut-off value for determining complications was estimated to be 4.6%.
DISCUSSION
In this study, we retrospectively investigated the association between preoperative 3D-CT volumetry and postoperative complications in lung cancer patients. The LAV% calculated from 3D-CT volumetry was significantly correlated with complications after lobectomy, as well as with PPS by multivariate logistic regression analysis. Preoperative FEV1% on spirometry was also correlated with postoperative complications by univariate analysis, while it was not significantly correlated with postoperative complications by multivariate analysis. These results suggest that the LAV% might be more sensitive for predicting complications than the FEV1%.
High-resolution CT is used to assess visually obstructive disease; low-attenuation areas on CT reflect emphysema or air trapping in COPD patients.14-16) Recently, CAD has enabled volumetry of low-attenuation areas from pulmonary 3D-CT data, and LAV% is frequently used to objectively evaluate the severity of emphysema.8, 10, 17, 18) Moreover, the present results demonstrated that the LAV% could be used as an imaging biomarker to predict postoperative complications. However, FEV1% has always been used to evaluate preoperative pulmonary function as a simple index of obstructive pulmonary disorder, even though it is affected not only by simple pulmonary function but also by other factors, including patient effort, age, and breathing muscle function, as well as the examiner’s skill. Mild obstructive pulmonary disorder might also play a role. In fact, one-third of the patients with an LAV% >2.2% in this study showed normal FEV1% values.
We also evaluated suitable cut-off values for determining postoperative complications and PPS by ROC analysis, which were 2.2% and 4.6%, respectively. These values were unexpectedly low, primarily due to the fact that we used 5-mm–thick CT images and therefore may have underestimated partial volume effects. However, even if emphysema is mild, it has the potential to influence pulmonary function, particularly in the perioperative period since pulmonary volume reduction and inflammation occur after lobectomy.
Patient age was also a significant factor for postoperative complications. Male gender and smoking history, which are known to be associated with COPD, were significantly correlated with postoperative complications in univariate analysis, but not in multivariate analysis. LAV% was more closely correlated with respiratory function. Neither BMI nor the resected lobe were significantly correlated with postoperative complications or PPS. The present results are consistent with the reports of Smith et al. and Dhakal et al., which stated that obesity does not increase the incidence of perioperative complications or length of stay following anatomic resection for non-small cell lung cancer.19, 20)
Several studies have shown that preoperative respiratory rehabilitation programs and use of inhaled bronchodilators in lung cancer patients with COPD can effectively reduce postoperative complications.21-25) Traditionally, COPD is diagnosed based on FEV1% values measured by spirometry. However, the present results showed that LAV% values calculated by 3D-CT could more correctly predict COPD compared to FEV1%. Therefore, preoperative affirmative intervention based on LAV% in lung cancer patients might be more effective for preventing postoperative complications.
This study has four limitations. First, it is a retrospective and single-center study. Second, we used 5-mm–thick images to reconstruct 3D-CT, which caused some inaccuracies in the calculation of LAV, as noted above. We suggest that recently developed high-speed CT scanners that can acquire sequential sub-millimeter images and thus more correctly calculate LAV be used in future analyses. Third, we could not evaluate wall thickness of bronchi, which represents airway inflammation, due to the low resolution of the 5-mm–thick CT images. COPD consists of emphysema and obstructive bronchitis, and bronchial wall thickness is significantly correlated with airway obstruction.26) To predict postoperative complications more accurately, additional analysis of the bronchial wall is needed. Fourth, we could not evaluate the difference of LAV distribution between upper and lower lobes because the interlobar fissure was fuzzy on 5-mm thickness CT images. Recent studies show that pulmonary function seem to be different between the upper and lower lobes in COPD patients.7, 27) Further investigation is needed.
In conclusion, we have demonstrated that LAV% on preoperative chest 3D-CT is significantly associated with postoperative complications and PPS. Multivariate analysis showed that age and LAV% were significantly correlated with postoperative complications, and that LAV% was significantly correlated with PPS. A suitable LAV% cut-off value for determining postoperative complications and PPS were estimated to be 2.2% and 4.6%, respectively. LAV% was more sensitive for predicting complications after lobectomy for lung cancer than FEV1%, and could potentially be used as a biomarker in the implementation of preoperative respiratory rehabilitation programs.
ACKNOWLEDGEMENT
We thank an experienced medical editor in JAM Post Inc. for English check and revision.
Conflict of interest: None declared
Funding: None declared
Abbreviations
- 3D-CT
Three-dimensional computed tomography
- AUC
area under the curve
- BI
Brinkman index
- BMI
body mass index
- CAD
computer-aided diagnosis
- COPD
chronic obstructive pulmonary disease
- FEV1
forced expiratory volume in 1 second
- FEV1%
forced expiratory volume in 1 second as percent forced vital capacity
- GOLD
the global initiative for chronic obstructive lung disease
- HU
Hounsfield units
- LAV
low-attenuation volume
- LAV%
percentage of low-attenuation volume
- PFT
pulmonary function test
- PPS
prolonged postoperative stay
- POT
prolonged oxygen treatment
- PV
prolonged ventilation
- ROC
receiver operating characteristic
- SD
standard deviation
- TLV
total lung volume
REFERENCES
- 1).Raviv S, Hawkins KA, DeCamp MM, Jr., Kalhan R. Lung cancer in chronic obstructive pulmonary disease: enhancing surgical options and outcomes. Am J Respir Crit Care Med, 2011; 183: 1138–1146. [DOI] [PubMed]
- 2).Smetana GW. Preoperative pulmonary evaluation: identifying and reducing risks for pulmonary complications. Cleve Clin J Med, 2006; 73 Suppl 1: S36–41. [DOI] [PubMed]
- 3).Smetana GW. Preoperative pulmonary evaluation. N Engl J Med, 1999; 340: 937–944. [DOI] [PubMed]
- 4).Licker MJ, Widikker I, Robert J, Frey JG, Spiliopoulos A, Ellenberger C, Schweizer A, Tschopp JM. Operative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg, 2006; 81: 1830–1837. [DOI] [PubMed]
- 5).Jenkins C, Rodriguez-Roisin R. Quality of life, stage severity and COPD. Eur Respir J, 2009; 33: 953–955. [DOI] [PubMed]
- 6).Iwano S, Okada T, Satake H, Naganawa S. 3D-CT volumetry of the lung using multidetector row CT: comparison with pulmonary function tests. Acad Radiol, 2009; 16: 250–256. [DOI] [PubMed]
- 7).Matsuo K, Iwano S, Okada T, Koike W, Naganawa S. 3D-CT lung volumetry using multidetector row computed tomography: pulmonary function of each anatomic lobe. J Thorac Imaging, 2012; 27: 164–170. [DOI] [PubMed]
- 8).Matsuoka S, Yamashiro T, Washko GR, Kurihara Y, Nakajima Y, Hatabu H. Quantitative CT assessment of chronic obstructive pulmonary disease. Radiographics, 2010; 30: 55–66. [DOI] [PubMed]
- 9).Arakawa A, Yamashita Y, Nakayama Y, Kadota M, Korogi H, Kawano O, Matsumoto M, Takahashi M. Assessment of lung volumes in pulmonary emphysema using multidetector helical CT: comparison with pulmonary function tests. Comput Med Imaging Graph, 2001; 25: 399–404. [DOI] [PubMed]
- 10).Barbosa EM, Song G, Tustison N, Kreider M, Gee JC, Gefter WB, Torigian DA. Computational analysis of thoracic multidetector row HRCT for Segmentation and quantification of small airway air trapping emphysema in obstructive pulmonary disease. Acad Radiol, 2011; 18: 1258–1269. [DOI] [PubMed]
- 11).Ueda K, Kaneda Y, Sudoh M, Mitsutaka J, Tanaka N, Suga K, Hamano K. Role of quantitative CT in predicting hypoxemia and complications after lung lobectomy for cancer, with special reference to area of emphysema. Chest, 2005; 128: 3500–3506. [DOI] [PubMed]
- 12).Kroenke K, Lawrence VA, Theroux JF, Tuley MR, Hilsenbeck S. Postoperative complications after thoracic and major abdominal surgery in patients with and without obstructive lung disease. Chest, 1993; 104: 1445–1451. [DOI] [PubMed]
- 13).Matsuo M, Hashimoto N, Usami N, Imaizumi K, Wakai K, Kawabe T, Yokoi K, Hasegawa Y. Inspiratory capacity as a preoperative assessment of patients undergoing thoracic surgery. Interact Cardiovasc Thorac Surg, 2012; 14: 560–564. [DOI] [PMC free article] [PubMed]
- 14).Goddard PR, Nicholson EM, Laszlo G, Watt I. Computed-Tomography in Pulmonary-Emphysema. Clin Radiol, 1982; 33: 379–387. [DOI] [PubMed]
- 15).Bergin C, Muller N, Nichols DM, Lillington G, Hogg JC, Mullen B, Grymaloski MR, Osborne S, Pare PD. The Diagnosis of Emphysema - a Computed Tomographic Pathological Correlation. Am Rev Respir Dis, 1986; 133: 541–546. [DOI] [PubMed]
- 16).Sanders C, Nath PH, Bailey WC. Detection of emphysema with computed tomography. Correlation with pulmonary function tests and chest radiography. Invest Radiol, 1988; 23: 262–266. [DOI] [PubMed]
- 17).Iwano S, Kitano M, Matsuo K, Kawakami K, Koike W, Kishimoto M, Inoue T, Li YZ, Naganawa S. Pulmonary lobar volumetry using novel volumetric computer-aided diagnosis and computed tomography. Interact Cardiovasc Thorac Surg, 2013; 17: 59–65. [DOI] [PMC free article] [PubMed]
- 18).Tanabe N, Muro S, Oguma T, Sato S, Kiyokawa H, Takahashi T, Kudo M, Kinose D, Kubo T, Hoshino Y, Ogawa E, Hirai T, Mishima M. Computed tomography assessment of pharmacological lung volume reduction induced by bronchodilators in COPD. COPD, 2012; 9: 401–408. [DOI] [PubMed]
- 19).Smith PW, Wang H, Gazoni LM, Shen KR, Daniel TM, Jones DR. Obesity does not increase complications after anatomic resection for non-small cell lung cancer. Ann Thorac Surg, 2007; 84: 1098–1105. [DOI] [PubMed]
- 20).Dhakal B, Eastwood D, Sukumaran S, Hassler G, Tisol W, Gasparri M, Choong N, Santana-Davila R. Morbidities of lung cancer surgery in obese patients. J Thorac Cardiovasc Surg, 2013; 146: 379–384. [DOI] [PMC free article] [PubMed]
- 21).Bobbio A, Chetta A, Ampollini L, Primomo GL, Internullo E, Carbognani P, Rusca M, Olivieri D. Preoperative pulmonary rehabilitation in patients undergoing lung resection for non-small cell lung cancer. Eur J Cardiothorac Surg, 2008; 33: 95–98. [DOI] [PubMed]
- 22).Divisi D, Di Francesco C, Di Leonardo G, Crisci R. Preoperative pulmonary rehabilitation in patients with lung cancer and chronic obstructive pulmonary disease. Eur J Cardiothorac Surg, 2013; 43: 293–296. [DOI] [PubMed]
- 23).Kobayashi S, Suzuki S, Niikawa H, Sugawara T, Yanai M. Preoperative use of inhaled tiotropium in lung cancer patients with untreated COPD. Respirology, 2009; 14: 675–679. [DOI] [PubMed]
- 24).Nojiri T, Inoue M, Yamamoto K, Maeda H, Takeuchi Y, Nakagiri T, Shintani Y, Minami M, Sawabata N, Okumura M. Inhaled tiotropium to prevent postoperative cardiopulmonary complications in patients with newly diagnosed chronic obstructive pulmonary disease requiring lung cancer surgery. Surg Today, 2014; 44: 285–290. [DOI] [PubMed]
- 25).Suzuki H, Sekine Y, Yoshida S, Suzuki M, Shibuya K, Takiguchi Y, Tatsumi K, Yoshino I. Efficacy of perioperative administration of long-acting bronchodilator on postoperative pulmonary function and quality of life in lung cancer patients with chronic obstructive pulmonary disease. Preliminary results of a randomized control study. Surg Today, 2010; 40: 923–930. [DOI] [PubMed]
- 26).Nakano Y, Wong JC, de Jong PA, Buzatu L, Nagao T, Coxson HO, Elliott WM, Hogg JC, Pare PD. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med, 2005; 171: 142–146. [DOI] [PubMed]
- 27).Kundu S, Gu S, Leader JK, Tedrow JR, Sciurba FC, Gur D, Kaminski N, Pu J. Assessment of lung volume collapsibility in chronic obstructive lung disease patients using CT. Eur radiol, 2013; 23: 1564–1572. [DOI] [PMC free article] [PubMed]