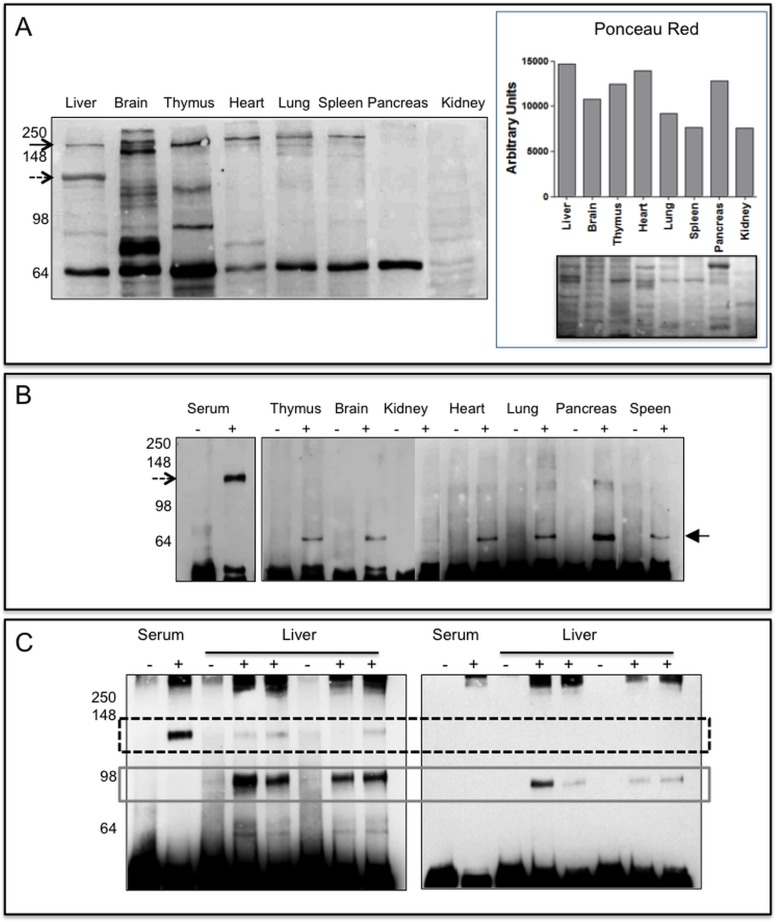
Fig 4. PTPRG expression in murine tissues.
Panel A (left): 20μg of lysates from perfused tissues were loaded on 10% SDS-PAGE, separated by gel electrophoresis and then blotted onto a PVDF membrane successively incubated with Rb anti-P4 antibody. Black arrow: full-length protein, dashed arrow highlights the 120 kDa protein detectable in liver lysates. On the right it is reported the densitometric analysis of the same samples stained with Ponceau Red and the corresponding image. Panel B: Immunoblotting reacted with Rb anti-P4 antibody of immunoprecipitated (IP) murine serum and tissue lysates (IP from 250 μg of tissue lysates). Lanes-: IP with Protein G Sepharose rabbit IgG isotype control. Lanes +: IP Protein G Sepharose Rb anti-P4. Dashed arrow: ∼120 kDa isoform, black filled arrow: ∼70 kDa isoform, maybe an intracellular, immature form of a soluble protein, or of a fragment of the full-length protein also present in Panel A. Lack of detectable amounts of full length protein is likely associated to residual proteolysis occurring during the IP step. Panel C: Immunoprecipitation from mouse serum and liver lysates performed with anti P4 antibody (+) or with RbIgG isotype (-). Left: WB performed with Rb anti-P4 reacting against an epitope located within the extracellular domain of human and murine PTPRG. Right: WB with polyclonal anti PTPRG (791–807 Abcam) reacting against an epitope located in the intracellular domain. Dashed box: ∼120 kDa and grey solid box: ∼90 kDa isoforms, respectively.