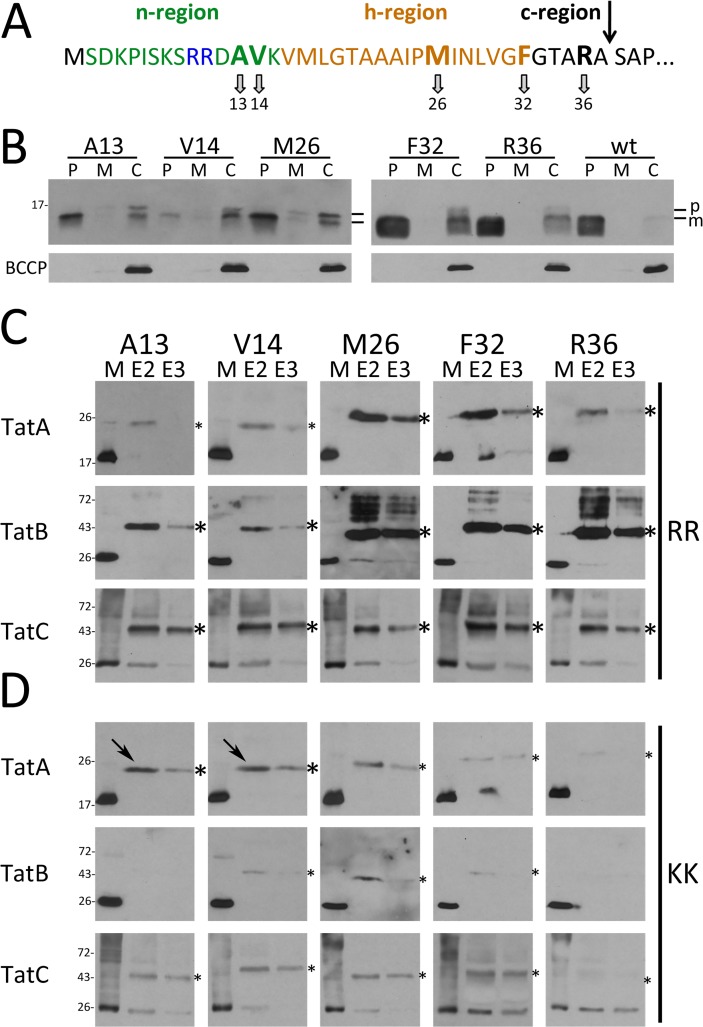
Fig 1. In vivo site-directed cross-linking of Tat signal peptides to the TatABC components.
(A) Amino acid sequence and positions of the pBpa cross-linker in the Tat signal peptide. The arrow indicates the signal peptide cleavage site. (B) Transport of native HiPIP and variants (produced from pEXH5-tac-H6 derivatives) with pBpa exchanges at indicated positions in pEvol-pBpF/pRK-tatABC-containing strains grown in the presence of 0.1 mM pBpa. Detection of HiPIP in subcellular fractions by SDS-PAGE/Western-blotting; the cytoplasmic biotin carboxyl carrier protein (BCCP; ∼22 kDa) was detected to control periplasmic purity. P: periplasm; M: membrane; C: cytoplasm; p: precursor HiPIP; m: mature HiPIP. (C) In vivo cross-links of twin-arginine signal peptides to Tat translocon components with pBpa at indicated positions. Specific cross-links are detected in affinity-chromatography elution fractions E2 and E3 by Western blotting using indicated Tat component-specific antibodies. Molecular weight standards are indicated at the left. (D) Effect of cross-links of RR>KK mutated signal peptides with pBpa exchanges; (strains and conditions in C) and D as in B)). Arrows indicate two TatA-cross-links with unaffected signal intensity. *, strong cross-links; *, weak cross-links; M, solubilized membrane fraction; E, elution fraction.