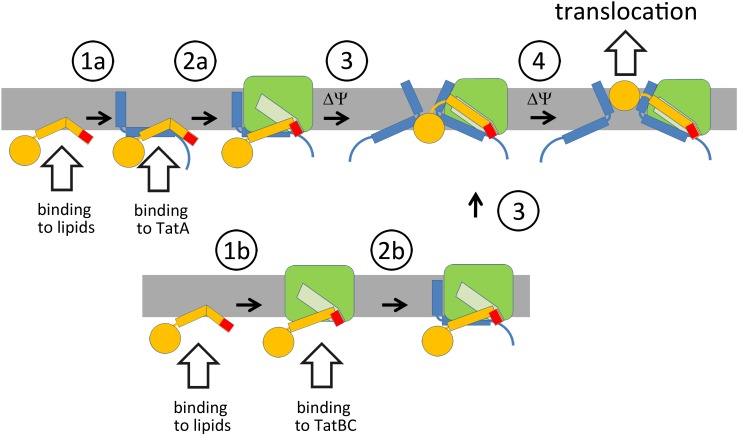
Fig 9. Interactions during Tat translocon assembly.
Tat substrate signal peptides such as from HiPIP or NrfC can spontaneously interact with membrane surfaces where they can either encounter free TatA (1a) or TatABC complexes (1b). TatA-bound substrates expose the RR-motif that is recognized by TatBC in the TatABC complex (2a). More TatA is recruited, possibly when TatBC imposes a force to the Tat substrate, which could influence TatA orientations and the local membrane curvature (3). Such a force could result from the known binding of larger signal peptide regions into the membrane-dunking binding-site regions at TatBC. Sufficient TatA recruitment finally permits translocation (4). If Tat substrates are first bound to TatBC, either after membrane interaction or directly (1b), the TatA assembly has to take place thereafter (2b). Such a binding may be more relevant for Tat substrates that do not readily interact with membranes, as the likelihood to first encounter TatA in membranes is high. “ΔΨ” designates steps that likely require the membrane potential. Color-code: Tat substrate, yellow; twin-arginine motif, red; TatA, blue; TatBC, green; binding-site for the signal peptide in TatBC, light green.