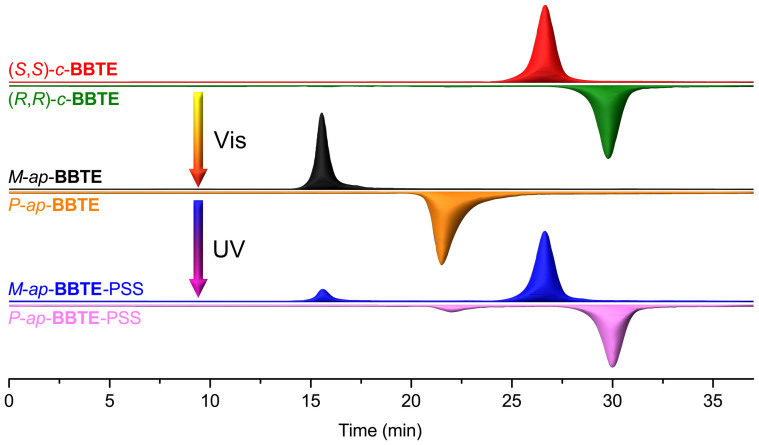
Figure 2. HPLC chromatogram of light-driven enantiospecific transformation of c-BBTE and ap-BBTE in CH3CN.
(R,R)- and (S,S)-c-BBTE were bleached with visible light (λ > 470 nm) to generate P- and M-ap-BBTE, and then irradiated with UV light (λ = 280 nm) to reach PSS, respectively. It is noted that (R,R)-c-BBTE, P-ap-BBTE, and PSS of P-ap-BBTE are vertically flipped for better comparison. Reversibly enantiospecific photochromism between photocyclization and cycloreversion can only proceed in [(S,S)-c-BBTE and M-ap-BBTE], and [(R,R)-c-BBTE and P-ap-BBTE]. Column: OD-R (CHIRALCEL® 4.6 diameter × 250 mm); Flow rate: 0.8 mL min−1; eluent: CH3CN/H2O (80/20, v/v); detecting wavelength: 303 nm (isobestic point).