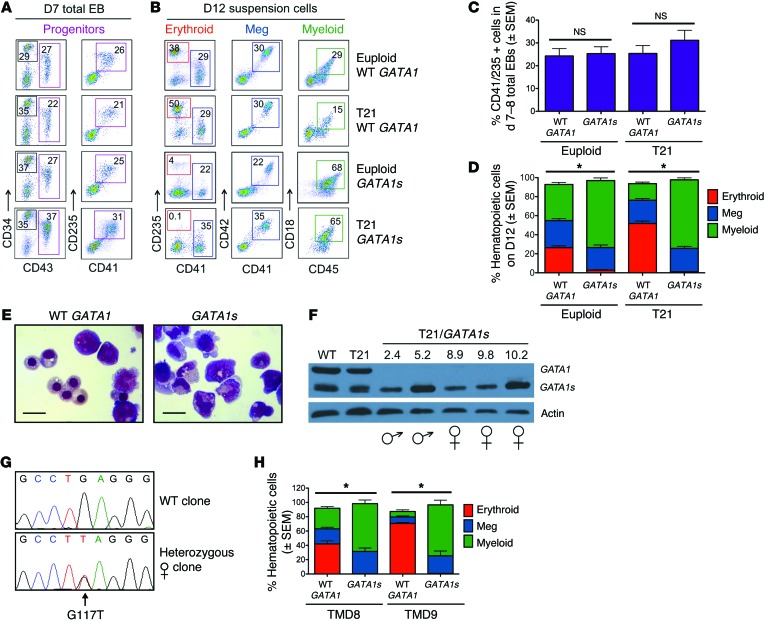
Figure 1. GATA1s mutations inhibit erythropoiesis from patient-derived iPSCs.
(A) Flow-cytometry analysis of CD34+/–CD43+CD41+CD235+ progenitors within total EB cultures on day 7 of hematopoietic differentiation and (B) suspension cells released from EBs on day 12 showing mature hematopoietic lineages: erythroid (CD41–CD235+), megakaryocytic (Meg, CD41+CD42+), and myeloid (CD45+CD18+). Numbers denote percentage of total cells in the indicated gate. (C) Frequency of CD43+CD41+CD235+ progenitor cells in EB cultures on days 7 and 8 of hematopoietic differentiation (n = 6; 17 independent experiments for euploid and T21 groups, respectively). (D) Summary of distribution of lineage-committed cells in EB suspension cultures on differentiation day 12 (n = 12; 20 independent assays for euploid and T21 groups, respectively). (E) Hematopoietic cell morphology on day 20 of differentiation cultures of isogenic WT GATA1 or GATA1s iPSCs. Scale bars: 50 μm. (F) Western blot of iPSC-derived hematopoietic cells. (G) DNA sequence analysis showing WT GATA1 and a heterozygous exon 2 mutation in 2 different iPSC clones from a female with DS and TMD. (H) Isogenic lines from 2 different TMD patients were analyzed. Percentages of mature lineages from day-12 EB suspension cultures, as in D. (n = 6; 4 independent assays for TMD8 and TMD9, respectively). *P < 0.005 for myeloid and erythroid lineages (2-tailed Student’s t test).