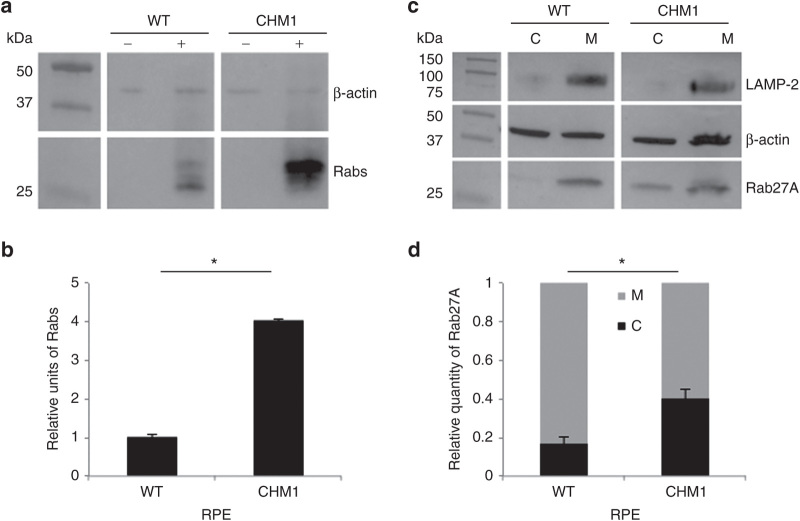
Figure 5.
Characterization of the biochemical defect in CHM1 RPE. (a) A representative in vitro prenylation assay using a biotinylated prenyl donor followed by western blot analysis shows a weaker signal of incorporated biotin for the wild-type RPE than for the CHM1 RPE. (b) Semiquantification of the biotinylated Rab pool, after normalization with β-actin loading, confirms a significantly lower (*P < 0.05) relative quantity of biotinylated cytosolic Rabs in the wild-type RPE as opposed to that in CHM1 (data expressed as mean ± SEM, n = 3). (c) Following differential centrifugation and western blot analysis of Rab27A expression in the cytosolic (C) and membrane (M) fractions, a lower amount of Rab27A is detected in the cytosol of wild-type RPE, compared with that in the membrane fraction, for an equal β-actin loading. A less striking difference in Rab27A content between the two fractions is observed in CHM1 RPE. The depletion of the membrane fraction from the cytosol was controlled by hybridization with an anti-LAMP-2 antibody. (d) Semiquantification of the cytosolic fraction of Rab27A versus the total cellular content confirms a significantly lower quantity (*P < 0.05) in wild-type (WT) RPE, as opposed to the quantity in CHM1 RPE (data expressed as mean ± SEM, n = 3). LAMP, lysosomal membrane–associated protein; RPE, retinal pigment epithelium.