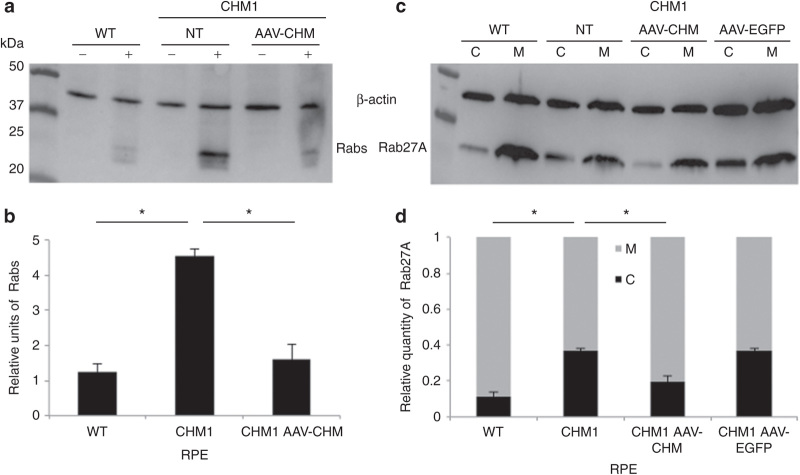
Figure 8.
Restoration of a normal cellular phenotype in CHM1 RPE following transduction with AAV2/5-CAG-CHM. (a) A representative in vitro prenylation, followed by western blot analysis of incorporated biotinylated prenyl donor in wild-type (WT), nontransduced (NT) CHM1 RPE, and CHM1 RPE transduced with 100,000 vector genomes (vg) per cell of AAV2/5-CAG-CHM. (b) Normalization to β-actin loading levels and semiquantification indicate that the biotinylated Rab content in wild-type RPE is significantly lower (*P < 0.05) than that of nontransduced CHM1 RPE (data expressed as mean ± SEM, n = 3). Following transduction of CHM1 RPE with AAV2/5-CAG-CHM, the biotinylated Rab pool was significantly reduced (*P < 0.05) to levels not significantly different from wild-type levels. (c) Differential centrifugation and western blot analysis of cytosolic and membrane fractions in wild-type (WT), nontransduced (NT) CHM1 RPE, and CHM1 RPE transduced with 100,000 vg per cell of AAV2/5-CAG-CHM or AAV2/5-CAG-EGFP. (d) Semiquantification analysis indicates that wild-type cytosolic Rab27A levels were significantly different (*P < 0.05) from the Rab27A levels in nontransduced CHM1. Following transduction of CHM1 RPE with AAV2/5-CAG-CHM, the cytosolic Rab27A content was significantly reduced (*P < 0.05) to levels not significantly different from the wild-type levels (data expressed as mean ± SEM, n = 3). By contrast, Rab27A cytosolic levels in CHM1 RPE were unchanged following transduction with AAV2/5-CAG-EGFP. AAV, adeno-associated virus; CAG, chicken β-actin with a CMV enhancer; RPE, retinal pigment epithelium.