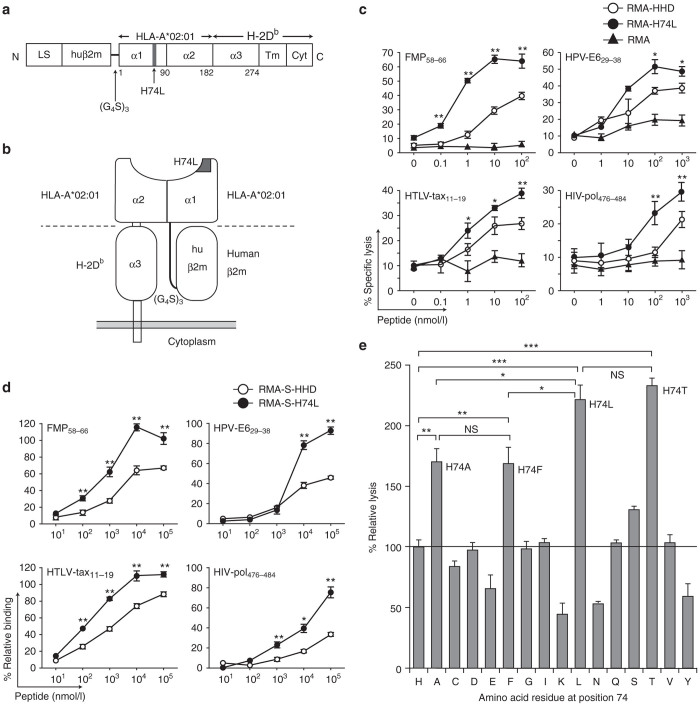
Figure 1.
HHD-H74L improves the presentation of exogenous peptides. (a) Structure of HHD-H74L. LS: leader sequence; huβ2m: human β2m; α1, α2, α3, Tm, Cyt: α1, α2, α3, transmembrane, cytoplasmic domains. (b) Diagram of HHD-H74L. (c) Enhanced cytotoxic T lymphocyte (CTL) recognition of HHD-H74L-expressing targets. CTL lines were examined for killing activities against RMA, RMA-HHD and RMA-H74L in the presence of a peptide (FMP58–66, HPV-E629–38, HTLV-tax11–19 or HIV-pol476–484) by 51Cr-release assays. Data are shown as the mean ± SD of triplicate wells. The experiments were repeated three times. *P < 0.05; **P < 0.01 compared to RMA-HHD, Student’s t-test. (d) Enhanced peptide-binding by HHD-H74L. RMA-S-HHD or RMA-S-H74L cells were cultured with a peptide, and examined for their surface expression of HHD or HHD-H74L by flow cytometry. The experiment was repeated twice with similar results. Data are standardized as the % relative binding, and shown as the mean ± SD of triplicate wells. *P < 0.05; **P < 0.01 compared to RMA-S-HHD, Student’s t-test. (e) CTL recognition of various HHD mutants. FMP-specific CTL lines were examined for killing activities against RMA expressing HHD mutants with various mutations at position 74 in the presence of 100 nmol/l FMP58-66 by 51Cr-release assays. The data are representative of one of three independent experiments. Data are standardized as the % relative lysis and shown as the mean ± SD of triplicate wells. *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant, One-way analysis of variance.