Abstract
Acute coronary syndromes (ACS) like unstable angina (UA) and acute myocardial infarction (AMI) can lead to the morbidity and mortality. The diagnosis and management of patients with ACS in the earliest times after symptom onset are considerably important in the emergency service. Study aimed to investigate the serum levels of heat shock protein 70 (Hsp 70), high sensitivity C-reactive protein (hsCRP), total creatine kinase (CK) activity, creatine kinase MB (CK-MB), cardiac troponin I (cTnl), leukocyte count (WBCs) and markers of oxidative stress in the first hours of ACS and to view their diagnostic values. 70 patients with ACS after admission and 20 sex-matched healthy controls were included in this study. Serum Hsp 70, hsCRP, CK, CK-MB, cTnl, protein carbonyls, malondialdehyde as well as whole blood WBCs were measured. The level of hsCRP was statistically higher in patients with AMI and UA than that of control group (p<0.001). WBCs and oxidized protein levels were higher in AMI than in UA and control groups. cTnl was related to CK-MB in AMI and UA groups (r=0.731, r=0.806, p<0.001, respectively) and also related with hsCRP in UA group (r=0.824, p<0.001). The mean Hsp 70 level was higher by 32.2% in AMI and 12.7% in UA patients compared to control subjects. hsCRP may have a role in the inflammatory response after ACS. In addition to cTnI and CK-MB, WBCs and hsCRP may be useful as a marker for the identification of ACS patients with chest pain in early diagnosing.
KEY WORDS: acute coronary syndromes, Hsp 70, hs-CRP, oxidative stress
INTRODUCTION
Acute coronary syndromes include unstable angina and acute myocardial infarction in which myocardial ischemia and/or necrosis trigger the inflammation and subsequent repair processes. Oxidative stress and chronic inflammatory responses play a mysterious role in the initiation and progression of ACS [1-3]. Plaque rupture and subsequent thrombosis at the site of the plaque rupture are the most common underlying pathophysiologic mechanisms of ACS [4,5]. The definition of acute coronary syndrome depends on the specific characteristics of each element of the triad of clinical presentation (including a history of coronary artery disease), biochemical cardiac markers such as creatine kinase-MB isoenzme, cardiac troponins and electrocardiographic changes. Patients with ACS span a large spectrum of risk that progresses from UA to non-ST-elevation myocardial infarction and to ST-elevation myocardial infarction [6]. Detecting patients in the early hours of ACS is still a challenge for emergency physicians. For instance, the ECG is often nondiagnostic for acute chest pain, and in fact, the sensitivity of the baseline ECG for detecting AMI is only 60%, and up to 33% of patients with ACS have no chest pain [7]. Misdiagnosis has been reported to be the main cause of treatment delays [8]. In last decade, routine serum markers (CK-MB, cTnI and T, myoglobin) of myocardial injury in acute coronary cases reflect only the abnormalities of the inflammatory milieu and plaque rupture. However, novel identified serum substances are the components of vascular inflammation and/or atherosclerotic plaque instability have drawn recent attention for their ability to portend acute clinical events and their outcomes. Within a number of markers, hsCRP and Hsp 70 are suggested as a risk marker of ACS in the literature [9-12]. Little is known about the utility of these biomarkers in combination and relationship with the other markers. In this study, we investigated the serum levels of hsCRP, Hsp 70, total CK activity, CK-MB, cTnI, WBCs and markers of oxidative stress (protein carbonyls, a marker of protein oxidation; malondialdehyde, a marker of lipid peroxidation) in the first hours of ACS in patients who were admitted to the Emergency Department (ED) for typical chest pain and diagnosed as ACS. In addition, we review the diagnostic values of CK-MB, cTnI, hsCRP, Hsp 70 and WBCs in view of ROC (receiver operation characteristic) curve analysis in the early assessment (ie, within 4-6 h of symptom onset) of suspected ACS in the ED and critically review oxidative stress in ACS patients.
MATERIALS AND METHODS
Patients and Procedures
The local ethical committee approved procedures used in this study. Patients with ACS who attended the ED of On-dokuz Mayis University Hospital were included in this prospective study after they had given informed consent. The patients with typical chest pain were enrolled in the study upon arrival to the ED within 4-6 h of the onset of symptoms. Typical chest pain was defined as squeezing pain over the precordial area radiating to the neck, arm, back or epigastric region accompanied by sweating, nausea, vomiting or syncope. Furthermore, this chest pain was no relieved by rest or no response by sublingual nitroglycerin. Atypical chest pain was defined as pleuritic, induced by palpation, confined to one point of the anterior chest wall, lasting only a few seconds or lasting for many hours [13]. Following initial clinical evaluation, all patients had a standard 12-lead ECG, and biochemical markers were assessed. Sex-matched twenty healthy volunteers with no chest pain or clinical evidence of heart disease served as controls. Histories, physical examination, chest radiography, ECG, exercise ECG testing and routine laboratory tests showed that the controls had no evidence of coronary heart disease. According to the changes in ECG findings and cardiac enzymes, patients were divided into two groups; a) myocardial infarction with ST elevation and without ST-elevation, and b) unstable angina pectoris. These groups were commonly classified under the category “acute coronary syndrome”. Patients with typical chest pain ongoing >30 min, 2 mm ST-elevation in at least two adjacent precordial leads, >1 mm in standard leads were classified as “ST-elevation myocardial infarction”. Patients without ST-elevation were classified as “non-ST-elevation myocardial infarction” or “unstable angina pectoris” according to the changes in CK-MB and cTnl levels [14]. When ACS was diagnosed, patients were admitted to the cardiac unit. All patients underwent coronary angiogram as a routine working up except healthy controls. Significant coronary artery disease was the presence of positive angiogram results at least 50% narrow in coronary artery.
Laboratory assays
Blood samples were taken from ACS patients upon arrival to the ED. Also, blood was taken from the control subjects. Leukocyte count was measured in the whole blood by LH-750 Beckman Coulter analyzer. The serum was separated by centrifugation from other venous blood sample, and then hsCRP, cTnI, total CK, and CK-MB (mass) analyzed by clinical chemistry laboratory and separated serum for the other tests stored at -80°C until analysis. Circulating hsp 70 was measured with ELISA kit (EKS-715, Stressgen) in the serum samples. Lipid peroxidation in serum was estimated spectro-photometrically by the tiobarbituric acid-reactive substance (TBARS) method with slight modifications and expressed in terms of malondialdehyde (MDA) [15]. Measurements of protein (carbonyl) oxidation in the serum samples were made by the method of Evans et al. [16] with slight modifications. Carbonyl concentration (nmol mg-1 of protein)= nmol ml-1 of carbonyl groups/protein concentration in mg ml-1.
Statistical analysis
All statistical analysis was performed with SPSS software (SPSS, release 13.0, Inc, Chicago, ILL). Data was tested for normality assumption. They were not normally distributed. Therefore non-parametric statistical analyses were used for all comparisons. Kruskal-Wallis test was used to determine the statistical significance of the differences in the groups. Then Mann-Whitney-U test (with Bonferroni correction) was used for comparisons between groups. ROC curves for the variables which have significant effect on ACS drawn to indicate the diagnostic values of test variables. Also, we calculated the Spearman Correlation Coefficient between the levels of serum parameters. We summarized continuous variables with mean ± standard deviation, categorical variables with percentages. At the 0.05 significance level the means of any two groups marked with the same letters indicate statistically non significant groups for each serum parameter.
RESULTS
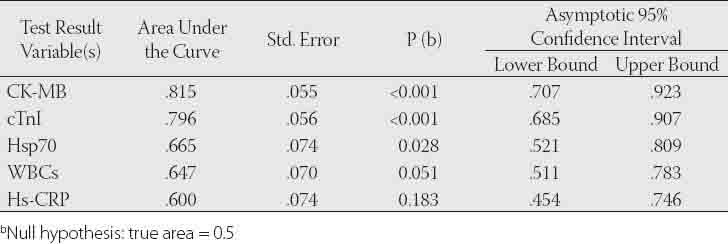
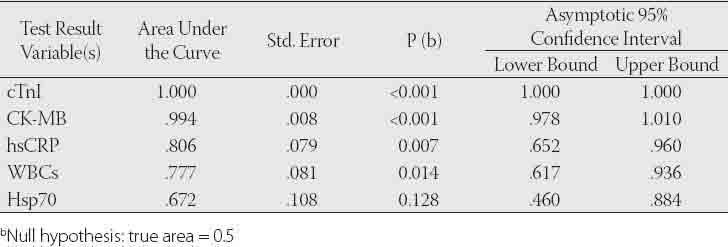
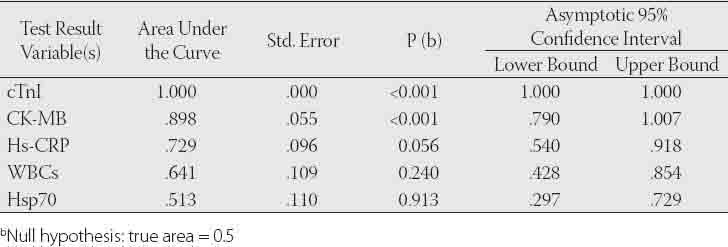
The general characteristics of the patients and control groups are shown in Table 1. As expected, all patients were more probably to have history of hypertension, diabetes, hyperlipidemia, smoking and family history of coronary heart disease than controls. As presented in Table 2, the mean level of Hsp 70 was higher by 32.2% in patients with AMI and 12.7% in patients with UA compared to control subjects. But no statistically difference was obtained for Hsp 70 levels among the groups (p>0.05). The levels of hsCRP in serum were significantly higher in patients with AMI and UA compared to control group (p<0.001). However, its mean value was found higher by 21.2% in patients with AMI when compared to UA group. Total CK levels were significantly elevated in patients with ACS as compared to levels in the control group (p<0.01). CK-MB and cTnI used to be the standard markers for diagnosing AMI; however, cTnI has proven more accurate in confirming or excluding AMI. The levels of these tests in serum were found significantly higher in patients with AMI when compared to control group (p<0.001). Also, CK-MB and cTnI levels were observed higher in patients with UA compared to control subjects. WBCs in AMI patients were measured over reference count (p<0.001). In addition, mean leukocyte counts in patients with UA were measured higher by 12.1% with respect to control group. Mean serum MDA was raised by 28,6% in patients with AMI and 47.1% in UA patients compared to control group. Similarly, serum protein carbonyls value was significantly increased in patients with AMI (p<0.05). However, its mean value was higher (by 7.1%) in patients with UA compared to control group. The Spearman’s correlation coefficients of CK-MB and cTnI were significant in AMI and UA groups (r=0.731, r=0.806, p<0.001, respectively). Also, the correlation coefficient between hsCRP and cTnI was significant (r=0.824, p<0.001). We found statistically significant correlations between WBC and hsCRP in AMI and UA groups (r=0.34, p=0.035, r=0.58, p=0.003, respectively). Diagnostic values for CK-MB, cTnI, Hsp 70, hsCRP and WBCs to differentiate the groups were shown in Figures 1, 2, 3 and their legends.
TABLE 1.
Characteristics and risk factor profile in all study groups.
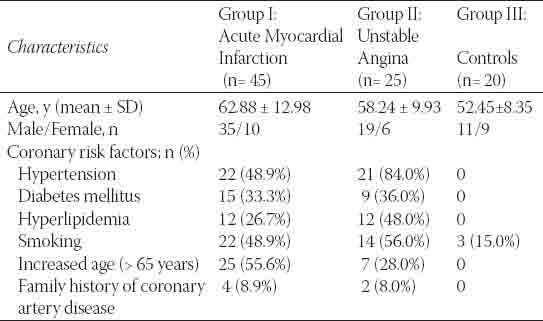
TABLE 2.
Serum Hsp 70, hsCRP, total CK, CK-MB, cTn I, WBCs, MDA and protein carbonyls levels in patients with coronary artery disease.
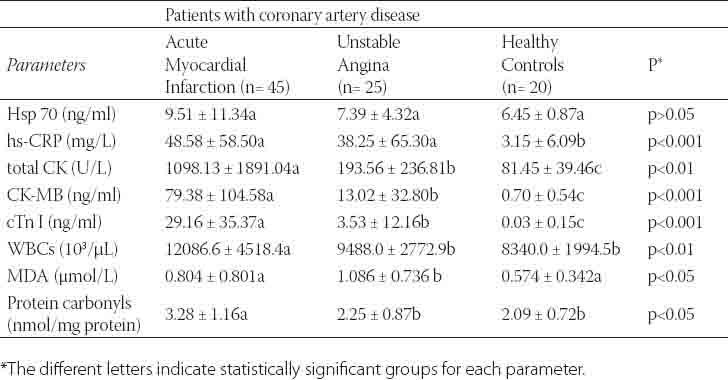
FIGURE 1.
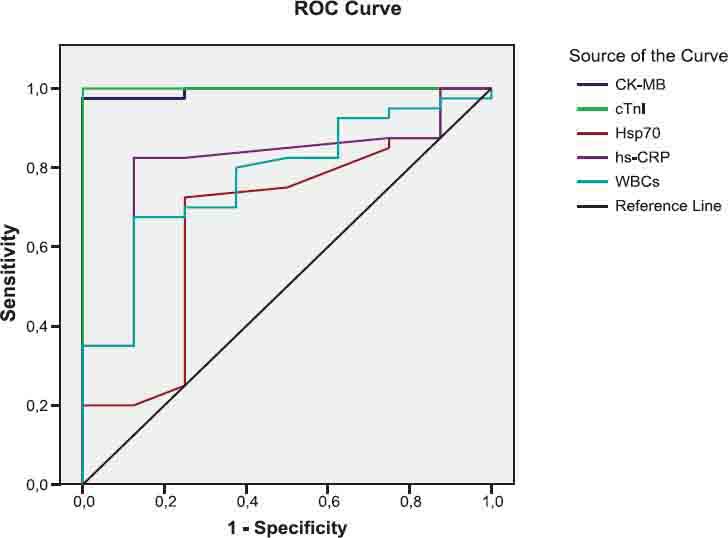
The diagnostic value of CK-MB, cTnl, Hsp70, hsCRP and WBCs tests to differentiate between AMI and UA groups
FIGURE 2.
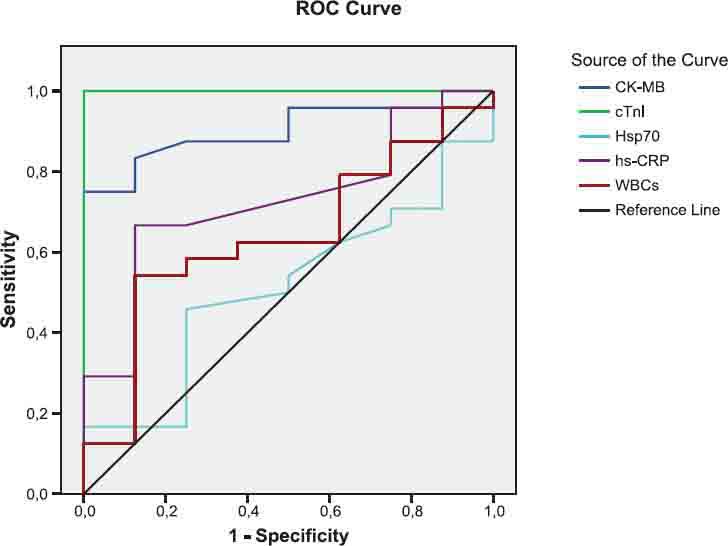
The diagnostic value of CK-MB, cTnl, Hsp70, hsCRP and WBCs tests to differen tiate between AMI and control groups.
FIGURE 3.
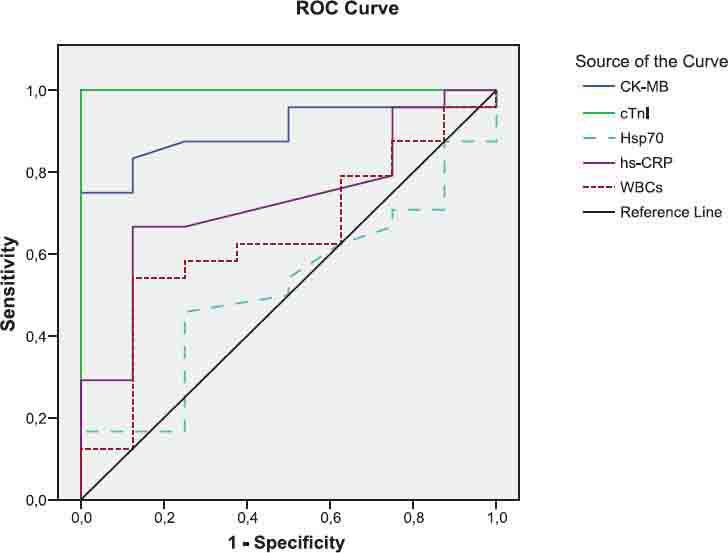
The diagnostic value of CK-MB, cTnI, Hsp70, hsCRP and WBCs tests to differentiate between UA and control groups.
DISCUSSION
Prompt recognition of a patient with an ACS is very important since appropriate therapy can markedly improve the prognosis of patients. New cardiac biomarkers have emerged as strong predictors of risk among patients presenting with ACS. Importantly, these biomarkers assess different pathophysiological mechanisms in myocardial ischemia: increases in cTnI indicate myocardial necrosis [17], CK-MB raises within 3-4 h of cardiac ischemia/necrosis [18], hsCRP, a marker of inflammation, is increased in patients with ACS [19]. Elevations in WBCs have been associated with the development of coronary artery disease and AMI [20]. Hsp 70 levels are independently associated with a higher risk of ACS [11]. Usually, it is accepted that CK-MB and cTnI are considerably important as AMI indicators in patients with ACS along with chest pain and ECG findings. In our study, statistically significant differences were obtained for the levels of these tests between the groups (Table 2). CK-MB and cTnI are gold markers in detecting AMI patients among subjects with ACS. CK-MB may set to differentiate between AMI and UA in the first hours of ACS as shown in Figure 1 and legend. By the way, these tests were observed to be an elevation in UA patients when compared to healthy control group. As a result of this finding, both test levels in patients with ACS might be sensitive signs to change from UA to AMI. Because during acute presentation of symptoms, there is a more contemporary continuum of events that begin with plaque rupture or erosion that lead to UA, and may progress to non-Q wave and Q-wave AMI at any moment [21]. Along with these explanations, patients with UA prior to AMI may be evaluated by CK-MB and cTnI leakage, and less Q-wave activity than patients with AMI of sudden onset [22]. Some UA patients with an abnormal CK-MB, cTnI and hsCRP may imply a transient myocardial ischemia in relation to duration ischemic episodes. Frequently, these patients are at high immediate risk for cardiac events. A number of studies have noted that the pathophysiology of coronary artery disease involves inflammation [9, 23, 24]. The hsCRP assays can detect low-grade inflammatory activity within the vascular system, which helps predict the first or recurrent coronary events [9]. In the present study, serum hsCRP levels were determined to be higher in UA patients, and highest in AMI patients with respect to control individuals. Interestingly, its value rises in parallel to the levels of increased CK-MB and cTnI in UA and AMI patients as indicated in Table 2. In prior studies, Tanaka et al. [25] reported that patients with plaque rupture at the culprit site showed higher hsCRP levels in the acute phase of AMI. Liuzzo et al. [26] showed that patients presenting with UA who had increased plasma levels of CRP (≥3 mg/L). A scientific statement of the Centers for Disease Control and Prevention/American Heart Association proposed a cutoff of 10 mg/L as more suitable when the predictive value of CRP was assessed in ACS [27]. According to our findings, an increased hsCRP level in UA and AMI, in the absence of systemic inflammatory disease, is a more likely to reflect widespread activation of inflammatory cells. Also, Hansson has indicated that the levels of C-reactive protein and interleukin-6 are elevated in patients with UA and AMI, with high levels predicting worse prognosis [28]. The use of hsCRP may significantly add to our ability to correctly identify patients presenting with ACS who are at high risk for future cardiovascular events. In addition to CRP, the other marker of inflammation and plaque instability is WBC count. Elevations in leukocyte count have been associated with adverse clinical outcomes and a higher mortality rate in the setting of ACS [29]. Indeed, platelet activation and markers of inflammation have been associated with coronary artery disease and ACS [20]. In our study, WBC count was measured high in patients presenting AMI, however it was elevated (by 12.1%) in UA patients with respect to reference count. Madjit et al. [30] reported that leukocytosis was an independent predictor of AMI. As a result of our study finding and literature reports [30, 31], leukocyte count in the first hours of ACS may be useful as diagnostic or prognostic indicator in patients with AMI or UA. On the other hand, an elevated leukocyte count and enhanced oxidative stress has been implicated in the pathogenesis of coronary artery disease and ACS [2, 3, 20, 32, 33]. Coronary atherosclerosis, the most common pathological process underlying cardiovascular disease, represents a state of heightened oxidative stress characterized by lipid and protein oxidation in vascular wall [34, 35]. In relation to this issue, findings of our study indicated that MDA was raised in the serum of UA patients and protein carbonyls in serum were increased in patients with AMI. Therefore, these results indicate the presence of oxidative stress in patients with ACS. Similarly, Serdar et al. [36] have demonstrated to increases serum lipid and protein oxidation in the first hours of ACS in patients with UA and AMI. Kostner et al. [32] have shown that lipid peroxidation in serum is significantly higher in UA as compared to patients with stable angina and to controls. Based on the results of scientific studies along with our findings, oxidative stress in ACS is an important event. It may lead to endothelial dysfunction and plaque disruption in patients. Heat shock proteins protect against cell damage and apoptosis. Their expression is up regulated upon exposure to stressful conditions such as ischemia, hypoxia, oxidative damage, mechanical shear stress, and inflammatory response. It has been shown that Hsp 70 protects cells against ischemic cardiac damage. Dybdahl et al. [12] had indicated that Hsp 70 was rapidly released into circulation after AMI. They have shown that circulating Hsp 70 is related to the extent of myocardial damage after admission to the emergency unit at six hours and suggested as a marker of myocardial injury. Valen et al. [22] reported that Hsp 70 (also called Hsp 72) was increased in atrial tissue of patients with UA. A recent work has demonstrated that plasma levels of Hsp 70 are markedly higher in patients with ACS within 12 h of the onset of symptoms than in the controls, and also Hsp 70 levels are higher in ACS than in stable angina [11]. However, mean serum level of Hsp 70 in the current study was higher in AMI and UA with respect to health controls. It seems to be Hsp 70 increases along with hsCRP in patients with ACS as shown in Table 2. Therefore, it might have a role in the inflammatory response against myocardial ischemia or necrosis.
CONCLUSIONS
As shown in Figures 1, 2, 3 and legends, ROC curve analyses indicated that CK-MB and cTnI provided the highest diagnostic accuracy for discriminating between patients in AMI and UA. After these tests, Hsp 70 has a higher diagnostic value for separating rather than WBCs and hsCRP (legend for Fig.1). The levels of cTnI and CK-MB have the highest diagnostic value for discriminating between individuals in AMI and health controls. According to ROC analysis results, WBCs and hsCRP have also high diagnostic accuracy with respect to control (legend for Figure 2). Patient population and healthy controls in this study are too small to allow any diagnostic and prognostic conclusions in ACS. Further investigation with large patient population and controls are required to confirm these findings in the prospective studies.
Legend for Fig. 1.
Legend for Fig. 2.
Legend for Fig. 3.
ACKNOWLEDGEMENTS
This study was supported partly by Ondokuz Mayis University Research Fund, T.430 project.
DECLARATION OF INTEREST
The authors declare no conflict of interest for present study.
REFERENCES
- [1].Inoue N, Kobayashi S, Yokoyama M. Oxidative stress and inflammation in plaque instability-role of vascular C-reactive protein. International Congress Series. 2004;1262:71–74. [Google Scholar]
- [2].Aukrust P, Berge RK, Ueland T, Aaser E, Damas JK, Wikeby L, et al. Interaction between chemokines and oxidative stress: possible pathogenic role in acute coronary syndromes. Journal of the American College of Cardiology. 2001;37(2):485–491. doi: 10.1016/s0735-1097(00)01110-4. [DOI] [PubMed] [Google Scholar]
- [3].Singh RB, Niaz MA, Sharma JP, Kumar R, Bishnoi I, Begom R. Plasma levels of antioxidant vitamins and oxidative stress in patients with acute myocardial infarction. Acta Cardiol. 1994;49(5):441–452. [PubMed] [Google Scholar]
- [4].Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- [5].Fuster V, Badimon J, Chesebro JH, Fallon JT. Plaque rupture, thrombosis, and therapeutic implications. Haemostasis. 1996;26:269–284. doi: 10.1159/000217308. [DOI] [PubMed] [Google Scholar]
- [6].Armstrong EJ, Morrov DA, Sabatine MS. Inflammatory biomarkers in acute coronary syndromes: part I: introduction and cytokines. Circulation. 2006;113(6):e72–e75. doi: 10.1161/CIRCULATIONAHA.105.595520. [DOI] [PubMed] [Google Scholar]
- [7].Moe KT, Wong P. Current trends in diagnostic biomarkers of acute coronary syndrome. Ann Acad Med Singapore. 2010;39:210–215. [PubMed] [Google Scholar]
- [8].Dadkhah S, Sharain K, Sharain R, Kiabayan H, Foschi A, Zonia C, et al. The value of bedside cardiac multibiomarker assay in rapid and accurate diagnosis of acute coronary syndromes. Crit Pathw Cardiol. 2007;6:76–84. doi: 10.1097/HPC.0b013e318053d1c9. [DOI] [PubMed] [Google Scholar]
- [9].Futterman LG, Lemberg L. High-sensitivity C-reactive protein is the most effective prognostic measurement of acute coronary events. American Journal of Critical Care. 2002;11(5):482–486. [PubMed] [Google Scholar]
- [10].Burke AP, Tracy RP, Kolodgie F, Malkom GT, Zieske A, Kutys R, et al. Elevated C-reactive protein values and atherosclerosis in sudden coronary death. Circulation. 2002;105(17):2019–2023. doi: 10.1161/01.cir.0000015507.29953.38. [DOI] [PubMed] [Google Scholar]
- [11].Zhang X, Xu Z, Zhou L, Chen Y, He M, Cheng L, et al. Plasma levels of Hsp 70 and anti-Hsp 70 antibody predict risk of acute coronary syndrome. Cell Stress and Chaperones. 2010;15(5):675–686. doi: 10.1007/s12192-010-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dybdahl B, Slordahl SA, Waage A, Kierulf P, Espevik T, Sundan A. Myocardial ischemia and the inflammatory response: release of heat shock protein 70 after myocardial infarction. Heart. 2005;91:299–304. doi: 10.1136/hrt.2003.028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cete Y, Eken C, Eray O, Goksu E, Kiyan S, Atilla R. The value of point-of-care fatty acid binding protein in patients with chest pain in determining myocardial infarction in the emergency setting. Hong Kong J Emerg Med. 2010;17:224–229. [Google Scholar]
- [14].Gururajan P, Gurumurthy P, Nayar P, Srinivasa Nageswara Rao G, Babu S, Cherian KM. Heart fatty acid binding protein (H-FABP) as a diagnostic biomarker in patients with acute coronary syndrome. Heart Lung Circ. 2010;19(11):660–664. doi: 10.1016/j.hlc.2010.06.665. [DOI] [PubMed] [Google Scholar]
- [15].Singh RP, Padmavathi B, Rao R. Modulatory influence of Adhatoda vesica leaf extract on the enzymes of xenobiotic metabolism, antioxidant status and lipid peroxidation in mice. Mol Cell Biochem. 2000;213:99–109. doi: 10.1023/a:1007182913931. [DOI] [PubMed] [Google Scholar]
- [16].Evans P, Lyras L, Halliwell B. Measurement of protein carbonyls in human brain tissue. Methods Enzymol. 1999;300:145–156. doi: 10.1016/s0076-6879(99)00122-6. [DOI] [PubMed] [Google Scholar]
- [17].Adams JE, 3rd, Bodor GS, Dávila-Román VG, Delmez JA, Apple FS, Ladenson JH, et al. Cardiac troponin I. A marker with high specificity for cardiac injury. Circulation. 1993;88:101–106. doi: 10.1161/01.cir.88.1.101. [DOI] [PubMed] [Google Scholar]
- [18].Dekker MS, Mosterd A, Hof AWJ, Hoes AW. Novel biochemical markers in suspected acute coronary syndrome: systemic review and critical appraisal. Heart. 2010;96:1001–1010. doi: 10.1136/hrt.2009.189886. [DOI] [PubMed] [Google Scholar]
- [19].Scirica BM, Morrow DA, Cannon CP, De Lemos JA, Murphy S, Sabatine MS, et al. Clinical application of C-reactive protein across the spectrum of acute coronary syndromes. Clinical Chemistry. 2007;53(10):1800–1807. doi: 10.1373/clinchem.2007.087957. [DOI] [PubMed] [Google Scholar]
- [20].Furman MI, Gore JM, Anderson FA, Budaj A, Goodman SG, Avezum A, et al. Elevated leukocyte count and adverse hospital events in patients with acute coronary syndromes: Findings from the Global Registry of Acute Coronary Events. Am Heart J. 2004;147:42–48. doi: 10.1016/j.ahj.2003.07.003. [DOI] [PubMed] [Google Scholar]
- [21].Wu AH, Ford L. Release of cardiac troponin in acute coronary syndromes: ischemia or necrosis? Clin Chim Acta. 1999;284:161–174. doi: 10.1016/s0009-8981(99)00078-9. [DOI] [PubMed] [Google Scholar]
- [22].Valen G, Hansson GK, Dumitrescu A, Vaage J. Unstable ungina activates myocardial heat shock protein 72, endothelial nitric oxide synthase, and transcription factors NFKB and AP-1. Cardiovascular Research 2000; 47:49–56. doi: 10.1016/s0008-6363(00)00071-7. [DOI] [PubMed] [Google Scholar]
- [23].Ross R. Atherosclerosis- an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- [24].Blake GJ, Ridker PM. C-raeactive protein and other inflammatory risk markers in acute coronary syndromes. J Am Coll Cardiol. 2003;41(4 Suppl S):37S–42S. doi: 10.1016/s0735-1097(02)02953-4. [DOI] [PubMed] [Google Scholar]
- [25].Tanaka A, Shimada K, Sano T, Namba M, Sakamota T, Nishida Y, et al. Multible plaque rupture and C-reactive protein in acute myocardial infarction. J Am Coll Cardiol. 2005;45(10):1594–1599. doi: 10.1016/j.jacc.2005.01.053. [DOI] [PubMed] [Google Scholar]
- [26].Liuzzo G, Biasucci LM, Gallimore JR, Grillo RL, Rebuzzi AG, Pepys MB, et al. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N Engl J Med. 1994;331:417–424. doi: 10.1056/NEJM199408183310701. [DOI] [PubMed] [Google Scholar]
- [27].Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;10(7):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- [28].Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- [29].Panteghini M. Role and importance of biochemical markers in clinical cardiology. European Heart Journal. 2004;25:1187–1196. doi: 10.1016/j.ehj.2004.04.026. [DOI] [PubMed] [Google Scholar]
- [30].Madjid M, Awan I, Willerson JT, Casscells SW. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol. 2004;44(10):1945–56. doi: 10.1016/j.jacc.2004.07.056. [DOI] [PubMed] [Google Scholar]
- [31].Menon V, Lessard D, Yarzebski J, Furman MI, Gore JM, Goldberg RJ. Leukocytosis and adverse hospital outcomes after acute myocardial infarction. Am J Cardiol. 2003;92:368–372. doi: 10.1016/s0002-9149(03)00651-9. [DOI] [PubMed] [Google Scholar]
- [32].Kostner K, Hornykewycz S, Yang P, Neunteufl T, Glogar D, Weidinger F, et al. Is oxidative stres causally linked to unstable angina pectoris? A study in 100 CAD patients and matched controls. Cardiovascular Research. 1997;36:330–336. doi: 10.1016/s0008-6363(97)00185-5. [DOI] [PubMed] [Google Scholar]
- [33].Misra MK, Sarwat M, Bhakuni P, Tuteja R, Tuteja N. Oxidative stres and ischemic myocardial syndromes. Med Sci Monit. 2009;15(10):209–219. [PubMed] [Google Scholar]
- [34].Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev 2004. 84(4):1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- [35].Stocker R, Keaney JF., Jr New insights on oxidative stress in the artery wall. J Thromb Haemost. 2005;3(8):1825–1834. doi: 10.1111/j.1538-7836.2005.01370.x. [DOI] [PubMed] [Google Scholar]
- [36].Serdar Z, Serdar A, Altin A, Eryilmaz U, Albayrak S. The relation between oxidant and antioxidant parameters and severity of acute coronary sydromes. Acta Cardiol. 2007;62(4):373–380. doi: 10.2143/AC.62.4.2022281. [DOI] [PubMed] [Google Scholar]


