Figure 8.
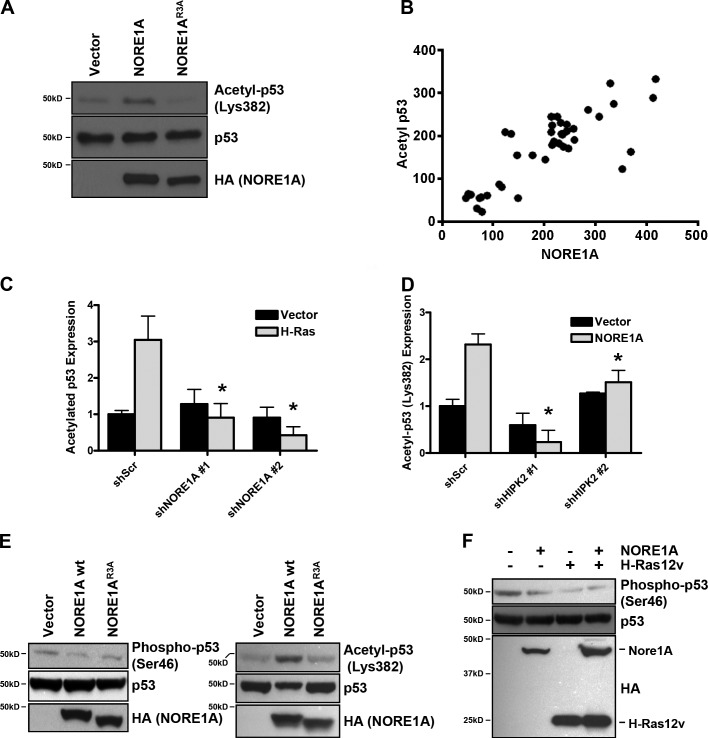
NORE1A mediates specific posttranslational modifications of p53 via HIPK2. (A) NORE1A promotes acetylation of p53 on residue K382. A549 cells were transfected with NORE1A WT or the HIPK2-defective NORE1AR3A mutant. The cells were lysed and immunoblotted for p53 acetylated at K382 using a K382-specific antibody. (B) NORE1A expression correlates with acetylated p53 expression in primary human tumor samples. The relationship between the protein levels (as assessed by Western blotting and quantification of the chemiluminescence signal) of NORE1A and those of acetylated p53 (K382) was measured in a collection of human HCC (n = 40) harboring WT p53. Axes are shown as relative luminescent units from samples with equal total protein loading. A significant, direct correlation was found between NORE1A and acetylated p53 levels. GraphPad Prism 5.01 was used to evaluate statistical significance by Tukey–Kramer and linear regression analyses (correlation coefficient [r] = 0.811, r2 = 0.6578, P < 0.0001). (C) Ras requires NORE1A to modulate p53 acetylation. HEK-293 cells were transiently transfected with the NORE1A shRNAs and activated H-Ras. Cells were lysed and assayed for endogenous p53 K382 acetylation after 24 h. Immunoreactive bands were quantified by densitometry and the results of 3 experiments plotted as a bar graph with data normalized to the scrambled control, *, P < 0.05 compared with cells transfected with the scrambled control. (D) NORE1A requires HIPK2 to modulate p53 acetylation. HEK-293 cells were transfected with NORE1A in the presence or absence of the HIPK2 shRNAs. After 24 h cells were lysed and assayed for endogenous p53 K382 acetylation. Immunoreactive bands were quantified by densitometry and the results of three experiments plotted as a bar graph, with data normalized to control cells transfected with the scrambled shRNA. *, P < 0.05 compared with cell transfected with the scrambled shRNA. (E) NORE1A suppresses S46 phosphorylation of p53. HEK-293 cells were transfected with expression constructs for NORE1A or the NORE1AR3A mutant. The cells were lysed and immunoblotted for endogenous p53 phosphorylated at S46 (left) or acetylated at K382 (right). (F) Ras suppresses p53 S46 phosphorylation. HEK-293 cells were transfected with expression constructs for NORE1A, activated H-Ras, or both. The level of S46 phosphorylation of endogenous p53 was then assayed by Western blot using a phospho S46–specific antibody. The Western blot shown is representative of three experiments. Total levels of endogenous p53 and transfected NORE1A/Ras are shown in the lower panels.