Abstract
Background
The aim of the study was to evaluate the dosimetric benefit of applying volumetric modulated arc therapy (VMAT) on the post-mastectomy left-sided breast cancer patients, with the involvement of internal mammary nodes (IMN).
Patients and methods
The prescription dose was 50 Gy delivered in 25 fractions, and the clinical target volume included the left chest wall (CW) and IMN. VMAT plans were created and compared with intensity-modulated radiotherapy (IMRT) plans on Pinnacle treatment planning system. Comparative endpoints were dose homogeneity within planning target volume (PTV), target dose coverage, doses to the critical structures including heart, lungs and the contralateral breast, number of monitor units and treatment delivery time.
Results
VMAT and IMRT plans showed similar PTV dose homogeneity, but, VMAT provided a better dose coverage for IMN than IMRT (p = 0.017). The mean dose (Gy), V30 (%) and V10 (%) for the heart were 13.5 ± 5.0 Gy, 9.9% ± 5.9% and 50.2% ± 29.0% by VMAT, and 14.0 ± 5.4 Gy, 10.6% ± 5.8% and 55.7% ± 29.6% by IMRT, respectively. The left lung mean dose (Gy), V20 (%), V10 (%) and the right lung V5 (%) were significantly reduced from 14.1 ± 2.3 Gy, 24.2% ± 5.9%, 42.4% ± 11.9% and 41.2% ± 12.3% with IMRT to 12.8 ± 1.9 Gy, 21.0% ± 3.8%, 37.1% ± 8.4% and 32.1% ± 18.2% with VMAT, respectively. The mean dose to the contralateral breast was 1.7 ± 1.2 Gy with VMAT and 2.3 ± 1.6 Gy with IMRT. Finally, VMAT reduced the number of monitor units by 24% and the treatment time by 53%, as compared to IMRT.
Conclusions
Compared to 5-be am step-and-shot IMRT, VMAT achieves similar or superior target coverage and a better normal tissue sparing, with fewer monitor units and shorter delivery time.
Keywords: breast cancer, radiotherapy, VMAT, IMRT
Introduction
Among the most commonly diagnosed cancers, breast cancer alone accounts for 29% of all new cancers among women in 2014.1 Most early-stage patients can be treated with breast conserving surgery, adjuvant radiotherapy or systemic treatment combined with neoadjuvant chemotherapy.2 However, patients with the advanced conditions usually receive mastectomy and postoperative radiotherapy. It has been shown that adjuvant post mastectomy radiotherapy (PMRT) is efficient in reducing locoregional recurrence rate, and improving 10-year overall survival rate in patients with lymph node-positive breast cancer.3,4–8
However, there is a dosimetric challenge to deliver an uniform target dose to the patient with three-dimensional conformal radiotherapy (3D-CRT) if internal mammary node (IMN) is involved, especially in the patients with left-sided breast cancer.9,10 In order to achieve better cosmetic results and decrease the toxicity in normal tissues, the intensity modulated radiation therapy (IMRT) has been widely implemented in the clinic to improve the target dose homogeneity and conformity for breast cancer treatment as well as spare the irradiation doses of normal tissues.11–13 Compared to the 3D-CRT, Van der Laan et al. reported that the IMRT technique improved the chest wall (CW) and IMN dose coverage and reduced the cardiac dose. Previously, we conducted a similar study in 30 patients with left-sided post-mastectomy breast cancer, and the results showed that the conformity index of IMRT was better than that of 3D-CRT and IMRT increased the low-dose volume of normal tissue.14,15
Volumetric modulated arc therapy (VMAT), a novel technique that delivers the radiation dose to the target in a single or multiple gantry rotations, has been used in the treatment of many cancers sites, such as prostate, head and neck, and Hodgkin lymphoma.16–20 Some dosimetric studies compared VMAT with other techniques in treating breast cancer patients.21,22 Also, one study compared the rapid arc (a VMAT technique), IMRT and modified wide-tangent techniques in the left-sided breast cancer and found that the rapid arc could achieve similar target coverage as IMRT but with better organ at risks sparing and shorter treatment time, though only one patient received mastectomy in their 5-patient study.23 To master the application of VMAT with better efficacy, we investigated the dosimetric difference between the VMAT and IMRT in patients with left-sided breast cancer in the present study.
Patients and methods
Patients
From April 2009, the first fifteen left-sided breast cancer patients (T3/4, metastatic axillary lymph nodes > 4) treated in our department, with the mean age of 48 years (39 to 58), were enrolled in the study. All patients had undergone post-mastectomy and Level I–II nodal dissection and received the combined chemotherapy with or without trastuzumab. Patients were set up on a breast board (Med-Tec Corporation, USA) with the sternum parallel to the table and the left arms elevated above their heads. The patient’s head turned to the right side. The radio-opaque markers were placed on the patient’s midline, mid axillary line, the inferior aspect of the clavicle head, the inferior border at 1 cm below the contralateral infra mammary fold and the superior aspect of the fourth rib. CT images were acquired from the level of mandible to the lung base on a large bore CT scanner (Philips Medical, Fitchburg, WI, USA) with a slice thickness of 5 mm. All the images were exported to the Pinnacle treatment planning system (Pinnacle3 version 9.0, Philips Radiation Oncology Systems, Andover, MA) for contouring and treatment planning.
Target definitions
The clinical target volume (CTV) of CW (CTVCW) and IMN (CTVIMN) was delineated according to the Radiation Therapy Oncology Group (RTOG) breast cancer consensus definitions. The CTVIMN was contoured from the superior aspect of the medial first rib to the forth one by encompassing the internal mammary/thoracic vessels. A margin of 10 mm was added to CTVCW and CTVIMN to define the planning target volume of CW (PTVCW) and IMN (PTVIMN). Total PTV (PTVtotal) consisted of PTVCW and PTVIMN. All the PTVCW, PTVIMN and PTVtotal were limited to the skin surface. The organs at risk were also outlined: the heart contoured from the first CT slice below the pulmonary artery to the apex inferiorly; the entire ipsilateral and contralateral lung contoured; and the contralateral breast outlined based on the visible breast parenchyma.
Treatments
The treatments were planned for delivery on an Elekta Synergy linear accelerator (Elekta Oncology System, Crawley, UK) with 1-cm width multileaf collimator (MLC). A 5 mm tissue-equivalent bolus was placed on the patient’s skin with the coverage of PTV and surgical scar to increase the skin dose. The dose was calculated using the collapse cone superposition convolution algorithm with inhomogeneity correction.
In the present dosimetric study, one step-and-shoot IMRT and one VMAT treatment plan were created for each patient within the Pinnacle treatment planning system with the same dose optimization objectives. The isocenter was placed at the center of the PTV. The prescription dose was 50 Gy in 25 fractions. The plan quality for both treatment techniques was evaluated against the following criteria: at least 95% of the PTV volume receiving 50 Gy, 95% of the prescription dose (V95%) covering at least 99% of the PTV volume; the hot spot defined as PTV receiving more than 110% of prescription dose as little as possible; less than 20% of the left lung to 20 Gy (V20); less than 10% of the heart to 30 Gy (V30); a minimized dose to the contralateral lung and breast since some of the beams could penetrate the patient’s right lung and right breast.
A step-and-shoot IMRT plan with 5 beams (300, 0, 40, 80 and 110 degree) was created for each patient. The optimization was performed using the direct machine parameter optimization (DMPO) technique with preset parameters of minimum 3 monitor units, minimum 3 cm2 segment area and maximum 50 segments. Before the final dose calculation, the MLC leaves were manually pushed outside of the patient’s skin by 1 cm if they blocked only the air part in the beam’s eye view.
The SmartArc in Pinnacle was used for the VMAT planning. One or two 200 degree partial arcs (gantry rotated from 310 to 150 degrees) and 15 degree collimator rotation were utilized to generate VMAT plans. A 4-degree resolution was used for the final dose calculation. For the purpose of fair plan comparison, several step-and-shoot IMRT and SmartArc VMAT plans were created for the initial 3 patients and the best IMRT and VMAT plans were selected for dose volume histogram (DVH) data analysis. Then, the optimization parameters for the best plans were used for the following patients and all the required DVH data were obtained.
The DVHs of the PTVtotal, PTVIMN, lungs, heart and contralateral breast were derived from the IMRT and VMAT plans. For the targets were calculated the D98 (the minimum dose received by 98% of the target volume), D2, mean dose, dose homogeneity index (HI), V90% (percentage of the PTV receiving at least 90% of the prescription dos e) and V95%. D98 and D2 were used to evaluate the minimal and maximal dose to the target, respectively. The homogeneity index was calculated as follows:
where the Dp is the prescription dose, and lower HI means better homogeneity. Additionally, the V110% and V115% for the PTVIMN were also recorded. For the critical structures, the mean dose, V30, V5, and V10 of the heart, and V20, V5, V10 and mean dose of the ipsilateral lung, V5 and mean dose of the contralateral lung and mean dose of the contralateral breast were calculated. Number of monitor units and treatment delivery time were also calculated. Dry runs were performed for all the plans.
Statistical analysis
The results were represented as mean ± standard deviation (SD). Statistical analysis was performed using SPSS 17.0 software (Chicago, IL, USA). The two-sided paired t test was used when the data-sets were normally distributed. Otherwise, datasets were compared by Wilcoxon Cox test. The p value less than 0.05 was considered statistically significant.
Results
Target coverage
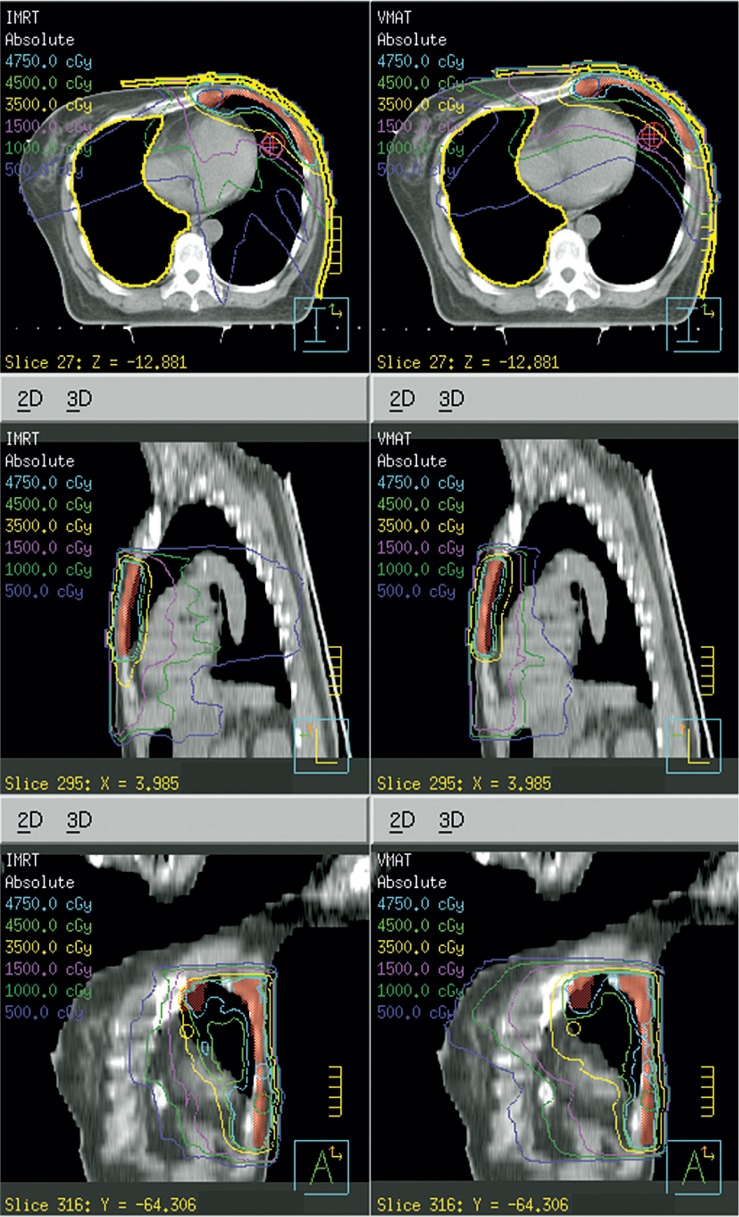
The mean volume of PTVtotal was 212cm3 (90 to 425 cm3) . A dose distribution is shown in Figure 1 and the corresponding DVHs in Figure 2 for a typical patient. The differences in the PTVtotal coverage and dose homogeneity between two techniques were of no statistical significance, V95% being 99.1% ± 1.1% with VMAT and 98.9% ± 1.1% with IMRT (p = 0.363); the similar maximum dose of PTVtotal defined as one in 2% of the target volume, i.e. D2, 55.6 ± 2.2 Gy with IMRT and 55.4 ± 1.7 Gy with VMAT, respectively; and the dose homogeneity index being 0.15 with both VMAT and IMRT (Table 1).
FIGURE 1.
Comparison between volumetric modulated arc therapy (VMAT) and intensity-modulated radiotherapy (IMRT) on dose distribution on the transverse plane at isocenter (from one representative case). The VMAT plan is on the right side and the IMRT on the left side.
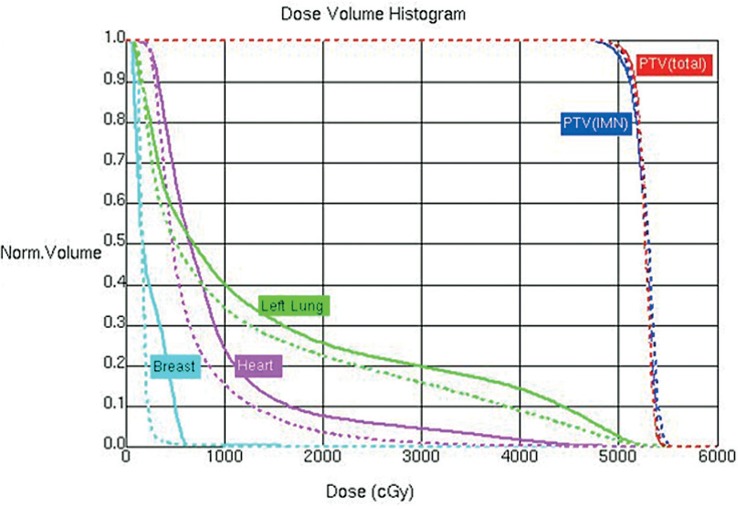
FIGURE 2.
Comparison between volumetric modulated arc therapy (VMAT) and intensity-modulated radiotherapy (IMRT) on dose volume histogram for PTVtotal, PTVIMN, heart, left lung and the contralateral breast (from one representative case shown in Figure 1). The VMAT plan is displayed as dashed line, IMRT plan as solid line.
TABLE 1.
Comparison of the dose coverage for the PTVtotal and the PTVIMN (mean ± SD)
| PTVtotal | IMRT | VMAT | p value | |
|---|---|---|---|---|
| Max dose (D2) | (Gy) | 55.6 ± 2.2 | 55.4 ± 1.7 | 0.760 |
| Min dose (D98) | (Gy) | 48.8 ± 1.0 | 48.5 ± 2.2 | 0.616 |
| Mean dose | (Gy) | 52.6 ± 1.2 | 52.4 ± 1.7 | 0.344 |
| HI | 0.15 ± 0.05 | 0.15 ± 0.01 | 0.602 | |
| V45 | (%) | 99.8 ± 0.3 | 100.0 ± 0.1 | 0.524 |
| V47.5 | (%) | 98.9 ± 1.1 | 99.1 ± 1.1 | 0.363 |
| PTVIMN | ||||
| Max dose (D2) | (Gy) | 56.8 ± 2.0 | 56.2 ± 1.6 | 0.126 |
| Min dose (D98) | (Gy) | 41.7 ± 5.4 | 45.3 ± 6.9 | 0.016 |
| Mean dose | (Gy) | 52.6 ± 1.8 | 53.1 ± 1.1 | 0.207 |
| HI | 0.15 ± 0.06 | 0.13 ± 0.06 | 0.048 | |
| V45 | (%) | 99.3 ± 1.5 | 100.0 ± 0.1 | 0.017 |
| V47.5 | (%) | 98.1 ± 2.9 | 99.2 ± 1.8 | 0.017 |
| V55 | (%) | 14.6 ± 24.6 | 15.7 ± 19.9 | 0.787 |
| V57.5 | (%) | 4.0 ± 16.3 | 2.0 ± 3.6 | 0.421 |
HI = homogeneity index; IMRT = intensity-modulated radiotherapy; Max = maximal; Min = minimal; PTVIMN = internal mammary node planning target volume; PTVtotal = planning target volume; SD = standard deviation; V45 = the percentage of the lung volume which receives radiation doses of 45 Gy; VMAT = volumetric modulated arc therapy
As for the dosimetric comparison data for the smaller PTVIMN, the VMAT plans provided a better IMN coverage than the IMRT ones, the mean values of V95% were 99.2% ± 1.8% and 98.1% ± 2.9% with VMAT and IMRT, respectively (p = 0.017). Although there was no significant difference in PTVIMN mean doses, the VMAT plans seemed to develop more homogeneous dose distribution in the IMN. The minimal dose to PTVIMN (D98) with VMAT was higher than that with IMRT (45.3 ± 6.9 Gy for VMAT vs 41.7 ± 5.4 Gy for IMRT) (p = 0.016). The mean HI was found to be 0.13 ± 0.06 with VMAT and 0.15 ± 0.06 with IMRT (p = 0.048). Both techniques presented comparable hot spots as the p values for V110% and V115% were 0.421 and 0.334, respectively (Table 1).
Normal tissue sparing
In terms of the doses to the normal tissues for the two treatment techniques, VMAT slightly reduced the mean dose to the heart, 13.5 ± 5.5 Gy for VMAT vs. 14.0 ± 5.3 Gy for IMRT (p = 0.792). Meanwhile, it did not show any significant differences in heart V30 and V5, as well as in V10 compared with IMRT (50.2% ± 29.0% with VMAT vs. 55.7% ± 29.6% with IMRT, p = 0.611) (Table 2).
TABLE 2.
Comparison parameters of normal tissue with VMAT or IMRT (mean ± SD)
| Structure | Parameters | IMRT | VMAT | VMAT/IMRT | p value | |
|---|---|---|---|---|---|---|
| Heart | Mean dose | (Gy) | 14.0 ± 5.3 | 13.5 ± 5.0 | 0.97 ± 0.05 | 0.792 |
| V30 | (%) | 10.6 ± 5.8 | 9.9 ± 5.9 | 0.91 ± 0.30 | 0.251 | |
| V10 | (%) | 55.7 ± 29.6 | 50.2 ± 29.0 | 0.89 ± 0.12 | 0.611 | |
| V5 | (%) | 77.0 ± 21.1 | 78.0 ± 20.1 | 1.02 ± 0.06 | 0.355 | |
| Left Lung | Mean dose | (Gy) | 14.1 ± 2.3 | 12.8 ± 1.9 | 0.91 ± 0.05 | 0.001 |
| V20 | (%) | 24.2 ± 5.9 | 21.0 ± 3.8 | 0.89 ± 0.09 | 0.002 | |
| V10 | (%) | 42.4 ± 11.9 | 37.1 ± 8.4 | 0.89 ± 0.09 | 0.001 | |
| V5 | (%) | 66.0 ± 15.5 | 61.1 ± 18.0 | 0.92 ± 0.07 | 0.001 | |
| Right Lung | Mean dose | (Gy) | 4.67 ± 0.93 | 4.49 ± 1.06 | 0.94 ± 0.14 | 0.409 |
| V5 | (%) | 41.2 ± 12.3 | 32.1 ± 18.2 | 0.71 ± 0.31 | 0.034 | |
| Right Breast | Mean dose | (Gy) | 2.3 ± 1.6 | 1.7 ± 1.2 | 0.70 ± 0.04 | 0.002 |
IMRT = intensity-modulated radiotherapy; SD = standard deviation; V20 = the percentage of the lung volume which receives radiation doses of 30 Gy; VMAT = volumetric modulated arc therapy
It was also found that the VMAT plans achieved lower mean dose to the left lung than the IMRT ones, i.e., 12.8 ± 1.9 Gy vs. 14.1 ± 2.3 Gy (p = 0.001). Moreover, the values of left lung V20, V10 and V5 were 21.0% ± 3.8%, 37.1% ± 8.4%, 61.1% ± 18.0% for VMAT, and 24.2% ± 5.9%, 42.4% ± 11.9 %, 66.0% ± 15.5% for IMRT. There was no significant difference in the mean dose of right lung, but VMAT plans achieved lower V5 to the right lung, as compared to IMRT (32.1% ± 18.2% with VMAT vs. 41.2% ± 12.3% with IMRT, p = 0.034). The mean dose to the contralateral breast was 1.7 ± 1.2 Gy and 2.3 ± 1.6 Gy, respectively (p = 0.001) (Table 2).
Monitor units and treatment delivery time
The dose rate for IMRT was 512 MU/min, and the maximum dose rate for VMAT was 512 MU/min. The mean number of MU for VMAT plans was 462 (range, 380 to 590 MU) compared to 604 (range, 488 to 850 MU) for IMRT. The mean treatment time for one arc was 2.0 minutes, and the mean treatment time to deliver two arcs was 4.20 minutes (range, 4.1 to 4.3 minutes) compared to 9.0 minutes (range, 8.7 to 11.2 minutes) for IMRT.
Discussion
IMRT and VMAT can shape the dose to the concave target in the CW and IMN in breast cancer radiotherapy. In the current study, we reported a dosimetric comparison between the two techniques on 15 cases of left-sided breast cancer. The step-and-shoot IMRT plans using DMPO technique and the VMAT plans using the SmartArc were used in the Pinnacle treatment planning system. In our study, CT images were acquired base on a CT scanner with a slice thickness of 5 mm. Though the widths of slices are usually 2–3 mm, CT scan could also be performed using 5 mm slice thickness to evaluate the dose distribution of IMRT3.3–7
Target coverage
It has a benefit in maximizing efficacy and improving local control to ensuring homogeneous dose coverage of PTV by avoiding areas of under dose (‘cold spots’, PTV receiving less than 90% of prescription dose), and at the same time eliminating areas of relative overdose (‘hot spots’), minimizing normal long-term tissue toxicity (skin changes and fibrosis) which negatively affect cosmesis. In our study, the IMRT and VMAT plans showed similar PTVtotal coverages and both avoided the hot spots successfully. However, the VMAT had a better dose homogeneity in the PTVIMN by reducing the “cold spot”, which might decrease the local recurrence in the IMN area.
The radiotherapy target volume includes the CW, sup raclavicular fossa and IMN with or without the axilla.24,25 Though the inclusion of the supraclavicular region in the post-mastectomy radiotherapy has an influence on the dose to the ipsilateral lung, it is still reasonable and significant to compare the dose coverage between IMRT and VMAT when the supraclavicular region was not considered for all the patients. On the other hand, PTV should be a few millimetres below the skin surface. In our study, the PTVs were limited to the skin surface due to that chest has thinner wall with a few millimetres and bolus was placed on the patient’s chest skin surface to increase the skin dose. Therefore, the skin could provide the dose we needed and it is unnecessary to subtract a few millimetres from the skin surface.
Organs at risk dose
It has been shown that the V20 and mean dose to the lung are good predictors for radiation induced lung toxicity.26 Also, an analysis of non-small-cell lung cancer has shown that the V5 is a significant cut off point for the subsequent development of pneumonitis.27 When it comes to the breast cancer, it was found that clinically significant pneumonitis was rare if the V20 of ipsilateral lung was less than 30% for breast cancer patients.28 It has been also reported that the complication rate could be expected to be 20% if more than 50% of the lung volume received 10 Gy.29 We selected V20 < 20% as a criterion since it is also an optimization parameter in our centre. We found that the VMAT plan had a significant reduction in the V20, V10, V5 or the mean dose in the left lung than IMRT. Also, the VMAT showed the superior or similar right lung sparing compared with IMRT. These results strongly suggested that the VMAT technique could achieve better sparing of the lung.
It has been reported that the use of 3D-CRT and IMRT techniques in the treatment of breast cancer could reduce the cardiac dose and cardiac mortality.30,31 However, the potential cardiac toxicity was increased dramatically owing to the widespread use of anthracyclines, taxanes and trastuzumab.32,33 Therefore, it is critical to limit the heart dose in patients, especially those with left-sided breast cancer. It has been reported that the heart V30 of IMRT was significantly lower than 3D-CRT levels for patients underwent left- sided mastectomy.14 Rudat et al. have found that IMRT significantly reduced the ipsilateral lung dose and heart dose in 20 subsequent post mastectomy breast cancer patients.34 Moreover, VMAT has been revealed to deliver lower doses to the ipsilateral breast and lung and offer better dose conformity than 3D-CRT technique for partial breast irradiation patients.35 In this study, the dose to the heart for IMRT and VMAT was similar.
The dose to the contralateral breast is another critical factor to consider, especially in younger women who received RT. Previous studies showed that there was an elevated long-term risk of developing the secondary contralateral breast cancer, and the mean dose to the contralateral breast was 3.2 Gy with RapidArc.23,36 In our study, a slightly lower mean dose of 1.7 Gy was observed with VMAT, which may be the results of different dose calculation algorithms or inhomogeneity correction in the two treatment planning systems. We also found that the average mean dose to the contralateral breast was 2.3 Gy in the IMRT, suggesting that VMAT might have dosimetric effect in reducing the risk of contralateral breast cancer occurrence.
Organ motion
It is well known that the respiration-induced target motion can lead to variation between the planned and delivered dose. A 10 mm margin was applied in the study for expanding the CTV to PTV. We then evaluated the intra-fraction motion of the chest wall using the fluoroscopic imaging on the simulator and found that the maximum displacement was around 3 mm. It’s been reported that the respiratory movements of the breast during normal breathing were negligible, and at 80% of the tidal capacity the mean displacement of the breast and chest wall from the exhale was less than 1 mm in the anterior and superior directions.37,38 The 5 mm margin may extend the PTV to the outside of skin. With limited segments of the step-and-shot IMRT plans (maximum 50 segments), the MLC leaves can be pushed outwards from the patient’s skin by 1 or 2 cm if only the air part in BEV was blocked. However, such manual adjustment is unfeasible in the VMAT plans. Therefore, the solutions with improved target coverage for possible changes in size and position of target and rest tissues caused by respiration or oedema are to use the third-party software to move the block-air MLC away from the skin, or manually add 10-mm tissue around the skin for optimization but removing it in the final dose calculation.39 Another clinical advantage of VMAT is that it generally takes fewer MUs to deliver a VMAT treatment than IMRT for the same plan quality. Our results showed that the MUs for the fifteen chest walls examined by VMAT plans were about 2/3 to 3/4 of those by IMRT plans. Obviously, fewer MUs are always favourable as to shorten the treatment delivery time and reduce the whole body dose.
Conclusions
Overall, our results showed that VMAT achieved similar or superior target coverage, better normal tissue sparing, fewer monitor units and shortened delivery time, as compared with 5-beam step-and-shot IMRT.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant No. 81072164)
Footnotes
Disclosure: No potential conflicts of interest were disclosed.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Caudell JJ, De Los Santos JF, Keene KS, Fiveash JB, Wang W, Carlisle JD, et al. A dosimetric comparison of electronic compensation, conventional intensity modulated radiotherapy, and tomotherapy in patients with early-stage carcinoma of the left breast. Int J Radiat Oncol Biol Phys. 2007;68:1505–11. doi: 10.1016/j.ijrobp.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 3.Chung CS, Harris JR. Post-mastectomy radiation therapy: translating local benefits into improved survival. Breast. 2007;16(Suppl 2):S78–83. doi: 10.1016/j.breast.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–55. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 5.Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–8. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 6.Ragaz J, Jackson SM, Le N, Plenderleith IH, Spinelli JJ, Basco VE, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956–62. doi: 10.1056/NEJM199710023371402. [DOI] [PubMed] [Google Scholar]
- 7.Moo T-A, El-Tamer M. Use of postmastectomy radiation therapy in the treatment of breast cancer. Breast Cancer Management. 2012;1:177–80. [Google Scholar]
- 8.Duraker N, Demir D, Bati B, Yilmaz BD, Bati Y, Caynak ZC, et al. Survival benefit of post-mastectomy radiotherapy in breast carcinoma patients with T1–2 tumor and 1–3 axillary lymph node(s) metastasis. Jpn J Clin Oncol. 2012;42:601–8. doi: 10.1093/jjco/hys052. [DOI] [PubMed] [Google Scholar]
- 9.Coskun M, Ozsahin M, Sozzi WJ, Tsoutsou P. Application of Tomotherapy in Breast Cancer Patients. In: Haydaroglu A, Ozyigit G, editors. Principles and practice of modern radiotherapy techniques in breast cancer. New York: Springer Science and Business Media; 2013. pp. 299–318. [Google Scholar]
- 10.Hjelstuen MH, Mjaaland I, Vikstrom J, Dybvik KI. Radiation during deep inspiration allows loco-regional treatment of left breast and axillary-, supraclavicular- and internal mammary lymph nodes without compromising target coverage or dose restrictions to organs at risk. Acta Oncol. 2012;51:333–44. doi: 10.3109/0284186X.2011.618510. [DOI] [PubMed] [Google Scholar]
- 11.Dogan N, Cuttino L, Lloyd R, Bump EA, Arthur DW. Optimized dose coverage of regional lymph nodes in breast cancer: the role of intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1238–50. doi: 10.1016/j.ijrobp.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 12.Beckham WA, Popescu CC, Patenaude VV, Wai ES, Olivotto IA. Is multibeam IMRT better than standard treatment for patients with left-sided breast cancer? Int J Radiat Oncol Biol Phys. 2007;69:918–24. doi: 10.1016/j.ijrobp.2007.06.060. [DOI] [PubMed] [Google Scholar]
- 13.Coles CE, Moody AM, Wilson CB, Burnet NG. Reduction of radiotherapy-induced late complications in early breast cancer: the role of intensity-modulated radiation therapy and partial breast irradiation. Part II--Radiotherapy strategies to reduce radiation-induced late effects. Clin Oncol (R Coll Radiol) 2005;17:98–110. doi: 10.1016/j.clon.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 14.van der Laan HP, Korevaar EW, Dolsma WV, Maduro JH, Langendijk JA. Minimising contralateral breast dose in post-mastectomy intensity-modulated radiotherapy by incorporating conformal electron irradiation. Radiother Oncol. 2010;94:235–40. doi: 10.1016/j.radonc.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Q, Chen JY, Hu WG, Guo XM. Modified partially wide tangents technique in post-mastectomy radiotherapy for patients with left-sided breast cancer. Chin Med J (Engl) 2010;123:2825–31. [PubMed] [Google Scholar]
- 16.Palma D, Vollans E, James K, Nakano S, Moiseenko V, Shaffer R, et al. Volumetric modulated arc therapy for delivery of prostate radiotherapy: comparison with intensity-modulated radiotherapy and three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:996–1001. doi: 10.1016/j.ijrobp.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 17.Verbakel WF, Cuijpers JP, Hoffmans D, Bieker M, Slotman BJ, Senan S. Volumetric intensity-modulated arc therapy vs. conventional IMRT in head-and-neck cancer: a comparative planning and dosimetric study. Int J Radiat Oncol Biol Phys. 2009;74:252–9. doi: 10.1016/j.ijrobp.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 18.Weber DC, Peguret N, Dipasquale G, Cozzi L. Involved-node and involved-field volumetric modulated arc vs. fixed beam intensity-modulated radiotherapy for female patients with early-stage supra-diaphragmatic Hodgkin lymphoma: a comparative planning study. Int J Radiat Oncol Biol Phys. 2009;75:1578–86. doi: 10.1016/j.ijrobp.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Teoh M, Clark CH, Wood K, Whitaker S, Nisbet A. Volumetric modulated arc therapy: a review of current literature and clinical use in practice. Br J Radiol. 2011;84:967–96. doi: 10.1259/bjr/22373346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhide SA, Nutting CM. Advances in radiotherapy for head and neck cancer. Oral Oncol. 2010;46:439–41. doi: 10.1016/j.oraloncology.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Sakumi A, Shiraishi K, Onoe T, Yamamoto K, Haga A, Yoda K, et al. Single-arc volumetric modulated arc therapy planning for left breast cancer and regional nodes. J Radiat Res. 2012;53:151–3. doi: 10.1269/jrr.11159. [DOI] [PubMed] [Google Scholar]
- 22.Shaitelman SF, Kim LH, Yan D, Martinez AA, Vicini FA, Grills IS. Continuous arc rotation of the couch therapy for the delivery of accelerated partial breast irradiation: a treatment planning analysis. Int J Radiat Oncol Biol Phys. 2011;80:771–8. doi: 10.1016/j.ijrobp.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Popescu CC, Olivotto IA, Beckham WA, Ansbacher W, Zavgorodni S, Shaffer R, et al. Volumetric modulated arc therapy improves dosimetry and reduces treatment time compared to conventional intensity-modulated radiotherapy for locoregional radiotherapy of left-sided breast cancer and internal mammary nodes. Int J Radiat Oncol Biol Phys. 2010;76:287–95. doi: 10.1016/j.ijrobp.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 24.Yang B, Wei XD, Zhao YT, Ma CM. Dosimetric evaluation of integrated IMRT treatment of the chest wall and supraclavicular region for breast cancer after modified radical mastectomy. Med Dosim. 2014;39:185–9. doi: 10.1016/j.meddos.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Krueger EA, Fraass BA, McShan DL, Marsh R, Pierce LJ. Potential gains for irradiation of chest wall and regional nodes with intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:1023–37. doi: 10.1016/s0360-3016(03)00183-4. [DOI] [PubMed] [Google Scholar]
- 26.Kimura T, Togami T, Takashima H, Nishiyama Y, Ohkawa M, Nagata Y. Radiation pneumonitis in patients with lung and mediastinal tumours: a retrospective study of risk factors focused on pulmonary emphysema. Br J Radiol. 2012;85:135–41. doi: 10.1259/bjr/32629867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang S, Liao Z, Wei X, Liu HH, Tucker SL, Hu CS, et al. Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non-small-cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT) Int J Radiat Oncol Biol Phys. 2006;66:1399–407. doi: 10.1016/j.ijrobp.2006.07.1337. [DOI] [PubMed] [Google Scholar]
- 28.Blom Goldman U, Wennberg B, Svane G, Bylund H, Lind P. Reduction of radiation pneumonitis by V20-constraints in breast cancer. Radiat Oncol. 2010;5:99. doi: 10.1186/1748-717X-5-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yorke ED, Jackson A, Rosenzweig KE, Braban L, Leibel SA, Ling CC. Correlation of dosimetric factors and radiation pneumonitis for non-small-cell lung cancer patients in a recently completed dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63:672–82. doi: 10.1016/j.ijrobp.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 30.Harris EE, Correa C, Hwang WT, Liao J, Litt HI, Ferrari VA, et al. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol. 2006;24:4100–6. doi: 10.1200/JCO.2005.05.1037. [DOI] [PubMed] [Google Scholar]
- 31.Lohr F, El-Haddad M, Dobler B, Grau R, Wertz HJ, Kraus-Tiefenbacher U, et al. Potential effect of robust and simple IMRT approach for left-sided breast cancer on cardiac mortality. Int J Radiat Oncol Biol Phys. 2009;74:73–80. doi: 10.1016/j.ijrobp.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 32.Yardley DA. Integrating bevacizumab into the treatment of patients with early-stage breast cancer: focus on cardiac safety. Clin Breast Cancer. 2010;10:119–29. doi: 10.3816/CBC.2010.n.016. [DOI] [PubMed] [Google Scholar]
- 33.Marinko T, Dolenc J, Bilban-Jakopin C. Cardiotoxicity of concomitant radiotherapy and trastuzumab for early breast cancer. Radiol Oncol. 2014;48:105–12. doi: 10.2478/raon-2013-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudat V, Alaradi AA, Mohamed A, Ai-Yahya K, Altuwaijri S. Tangential beam IMRT versus tangential beam 3D-CRT of the chest wall in postmastectomy breast cancer patients: a dosimetric comparison. Radiat Oncol. 2011;6:26. doi: 10.1186/1748-717X-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu J-J, Chang Z, Wu QJ, Yoo S, Horton J, Yin F-F. Impact of volumetric modulated arc therapy technique on treatment with partial breast irradiation. Int J Radiat Oncol Biol Phys. 2010;78:288–96. doi: 10.1016/j.ijrobp.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 36.Stovall M, Smith SA, Langholz BM, Boice JD, Jr, Shore RE, Andersson M, et al. Dose to the contralateral breast from radiotherapy and risk of second primary breast cancer in the WECARE study. Int J Radiat Oncol Biol Phys. 2008;72:1021–30. doi: 10.1016/j.ijrobp.2008.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chopra S, Dinshaw KA, Kamble R, Sarin R. Breast movement during normal and deep breathing, respiratory training and set up errors: implications for external beam partial breast irradiation. Br J Radiol. 2006;79:766–73. doi: 10.1259/bjr/98024704. [DOI] [PubMed] [Google Scholar]
- 38.Moran JM, Balter JM, Ben-David MA, Marsh RB, Van Herk M, Pierce LJ. Short-term displacement and reproducibility of the breast and nodal targets under active breathing control. Int J Radiat Oncol Biol Phys. 2007;68:541–6. doi: 10.1016/j.ijrobp.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicolini G, Fogliata A, Clivio A, Vanetti E, Cozzi L. Planning strategies in volumetric modulated arc therapy for breast. Medical physics. 2011;38:4025–31. doi: 10.1118/1.3598442. [DOI] [PubMed] [Google Scholar]