Fig 2. Model of the HIV-1 PR backbone in front (left) and side (right) view (structure taken from RSCB protein data bank, PDB ID: 1OHR).
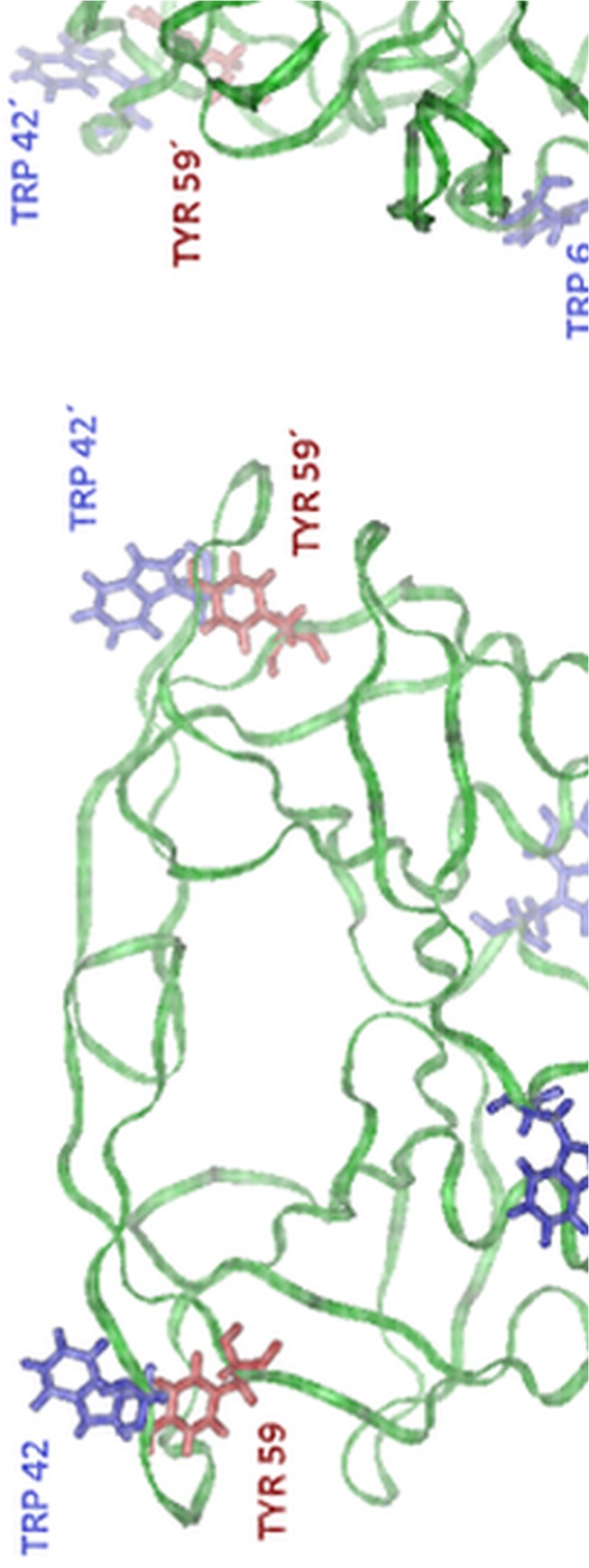
The residues of fluorescent amino acids, Trp6, Trp42 and Tyr59, are indicated. Trp6 is located close to the dimerization interface, therefore its spectroscopic properties are probably influenced by dimer dissociation. On the contrary, Trp42 and Tyr59 are located at the most distant site from the dimerization interface, therefore their fluorescence is probably unaffected by dimer dissociation, provided that the monomer keeps its conformation. Trp42 and Tyr59 can eventually partially influence this effect due to the change of their mutual configuration because the conformation of this part of the molecule undergoes partial changes with growing pressure, as was shown by the molecular-dynamics simulation [32]. All the fluorophores can contribute to the spectral changes induced by unfolding of monomers or protein aggregation when they undergo more extensive changes of their environment.
