Figure 1. βAPP signaling via the neurotrophic, amyloidogenic or phagocytic (clearance) pathways.
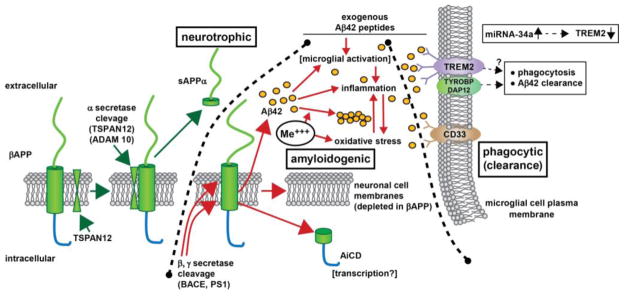
– beta amyloid precursor protein (βAPP) is a glycosylated integral trans-membrane protein of ~695–770 amino acids (~80 kDa MW) that is acted upon by a series of secretases to generate a range of neuroactive peptides. For example, α-secretase activity in association with tetraspanin (TSPAN12) proximity, distintegrin and metalloproteinase protein ADAM10, and other accessory proteins cleave βAPP to generate a ~621 amino acid neurotrophic sAPPα peptide via the neurotrophic signaling pathway. Interestingly sAPPα is very abundant in the vitreous of the eye and in healthy brain cells, particularly in the extracellular space (ECF) [Bhattacharjee, Zhao and Lukiw; unpublished observations]. Alternate processing of βAPP by tandem beta- and gamma secretase (β, γ secretase) cleavage (in the presence of ancillary membrane proteins such as presenilin 1 (PS1) and other proteins can result in the generation of 37–43 amino acid Aβ peptides, the most common of which are the peri-vascular Aβ40 and peri-neuronal Aβ42; the Aβ40 and especially Aβ42 peptides self-aggregate to form dense senile plaque and retinal drusen deposits (yellow ovals); the natural accumulation and aggregation of Aβ40 and especially Aβ42 peptides are strongly amyloidogenic; certain neurotoxic trivalent elements such as aluminum and iron (Me+++) and perhaps other genotoxic metals in our environment appear to stimulate this aggregation while also promoting oxidative stress, inflammation and other neurotoxic effects [84–90]. Importantly, Aβ42 peptides added exogenously and acutely may activate different cellular responses than the more chronic endogenous generation of these peptides [69,91–93,99,106]. Normally Aβ42 peptides are efficiently removed by a phagocytosis clearance system involving in part the triggering receptor for myeloid/microglial cells (TREM2), a variably glycosylated transmembrane sensor-receptor known to be enriched in the microglial cell plasma membrane. Signaling via the tyrosine kinase-binding protein (DNAX activation protein 12) [TYROBP (DAP12)] accessory receptor results in phagocytosis and ultimately, removal of Aβ42 peptides (yellow ovals) from the extracellular space [78–81]; interestingly, TREM2 knockout/knockdown mice have attenuated immunological and inflammatory responses and/or increases in age-related neuroinflammatory markers and cognitive deficiency [33,78,82]; TYROBP knockout mice exhibit immune system deficits and an impairment in microglial cell differentiation [68,78]; insufficient TREM2 may be in part responsible for the inability to adequately phagocytose Aβ42 peptides, resulting in their buildup and self-aggregation in the extracellular space. Aβ42 peptide sensing and clearance may also be accomplished in part through the microglial-enriched transmembrane CD33/Siglec protein and others (see text); interestingly, like TREM2, CD33/Siglec exhibits decreased expression in peripheral mononuclear cells in AD [99,100]. Upper right inset: miRNA-34a has been found to be significantly increased in AD hippocampal CA1, superior temporal lobe, in stressed microglial cells and in the retina of transgenic AD murine models overexpressing Aβ42 peptides [63,77; Bhattacharjee, Zhao, Lukiw, unpublished observations); miRNA-34a targeting of the TREM2 mRNA 3′-UTR and TREM2 down-regulation appears to be in part responsible for this defect [45,77–81,83,90,102,110]. Because miRNA-34a is encoded on an NF-kB-sensitive transcript, natural or synthetic anti-NF-kB and/or anti-miRNA strategies may be clinically useful in the restoration of homeostatic phagocytosis and the clearance of excessive Aβ42 peptides from the brain, CNS and retina in AD and AMD [39,74,75,111]. Black hatched lines between ‘neurotrophic’ and ‘amyloidogenic’ and ‘amyloidogenic’ and ‘phagocytic (clearance)’ are drawn to delineate specific βAPP cleavage product pathways, however, in brain and retinal physiology these pathways are most likely to be highly integrated to achieve homeostasis in the βAPP-sAPPα-Aβ42 trafficking system (see text).
