Figure 2.
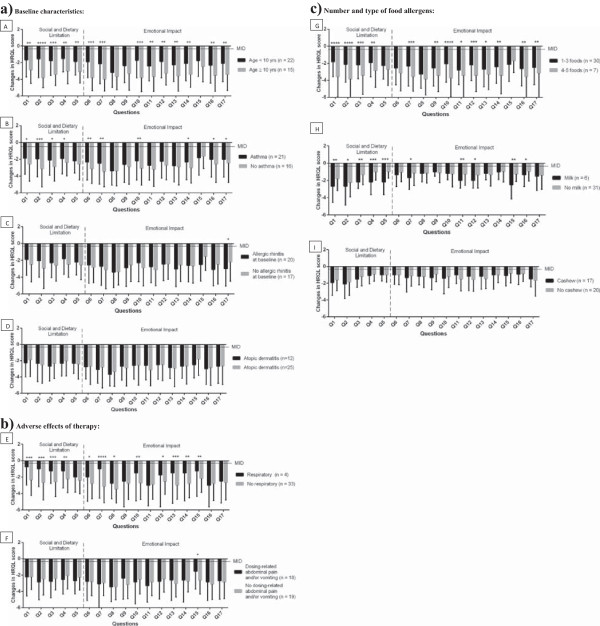
Changes in individual FAQL-PB question scores at 24-month follow-up time point from baseline for: a) baseline characteristics: (A) age, (B) asthma at baseline, (C) allergic rhinitis at baselineand (D) atopic dermatitis at baseline; b) adverse effects of therapy: (E) respiratory-related allergic reactions and (F) dosing- related abdominal pain and/or vomiting; c) number and type of food allergens: (G) 1-3 versus 4-5 foods; (H) milk; (I) cashew. Changes in individual FAQL-PB question scores are shown between caregivers of: (A) patients <10 years old and patients ≥10 years old; (B) patients with asthma and patients without asthma at baseline; (C) patients with allergic rhinitis and patients without allergic rhinitis at baseline; (D) patients with atopic dermatitis and without atopic dermatitis at baseline; (E) patients with at least one documented by a physician dosing- related respiratory adverse reaction (wheezing and/or cough) and patients without those; (F) patients with abdominal pain and/or vomiting and patients without abdominal pain and/or vomiting; (G) patients in treatment with 1-3 food allergens and patients in treatment with 4-5 food allergens; (H) patients in treatment with milk and patients not in treatment with milk; (I) patients in treatment with cashew and patients not in treatment with cashew. *p <0.05, **p <0.01, ***p <0.001, ****p <0.0001. Bars without asterisks represent non-significant changes.
