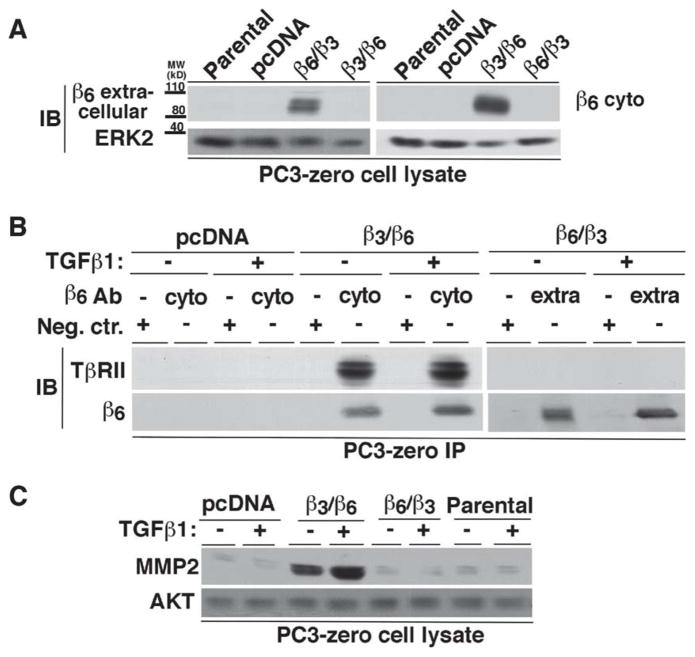
Figure 4. Association of TβRII with β6 through the β6 cytoplasmic domain induces MMP2.
(A) PC3-zero cells were transfected as follows: pcDNA, β6 extracellular and β3 cytoplasmic (β6/β3) or β3 extracellular and β6 cytoplasmic (β3/β6) chimera. Non-transfected PC3-zero cells are designated as Parental. Expression of either extracellular (left panel) or cytoplasmic (right panel) domain of β6 in Parental and PC3-zero transfectants was analyzed by IB. 2A1 Ab (10% SDS-PAGE under non-reducing conditions; left panel) was used to detect β6 extracellular domain or C-19 Ab (10% SDS-PAGE under reducing conditions; right panel) to detect β6 cytoplasmic domain. ERK was used as a loading control. (B) PC3-zero cells, transfected with pcDNA, β6/β3 integrin chimera or β3/β6 integrin chimera, were stimulated with 20 ng/ml TGFβ1 (48 h) or unstimulated. αvβ6 integrin was immunoprecipitated from lysates using either C-19 (which recognizes β6 cytoplasmic domain (cyto); left panel) or 10D5 (specific for β6 extracellular domain (extra); right panel) Ab. Goat IgG (left panel) or mouse IgG (right panel) was used as a negative control (Neg.Ctr.) Ab. The immunoprecipitates were analyzed by IB in order to detect TβRII. β6 integrin expression was also analyzed in the immunoprecipitates by using C-19 (10% SDS-PAGE under reducing conditions; left panel) or 2A1 (10% SDS-PAGE under non-reducing conditions; right panel) as described above. (C) PC3-zero cells were transfected with pcDNA, β6/β3 integrin chimera, β3/β6 integrin chimera or were non-transfected (Parental). Cells were serum starved for 24 h before being incubated with 20 ng/ml TGFβ1 for 48 h. Cell lysates were analyzed by 10% SDS-PAGE under reducing conditions and probed with an Ab to MMP2. AKT was used as a loading control.