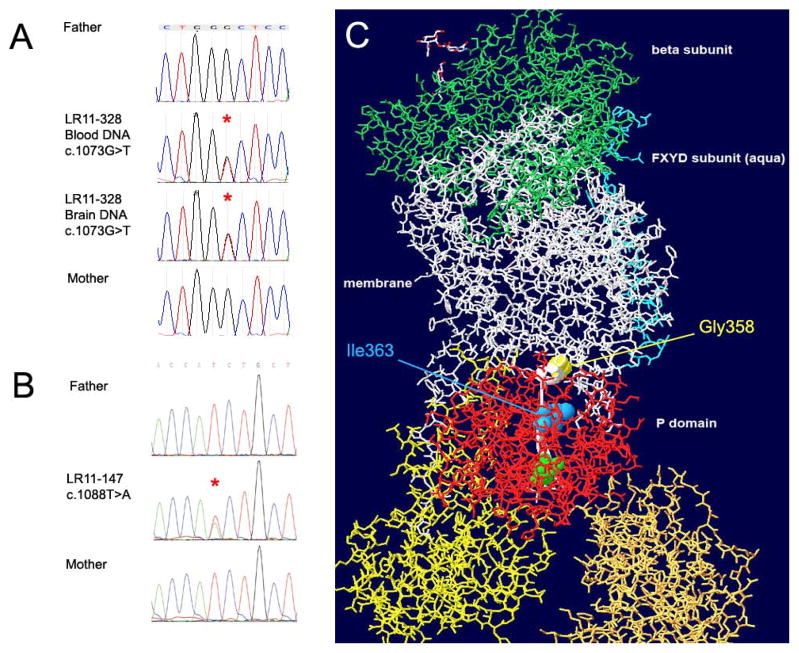
Figure 3.
Chromatographs of Sanger sequencing in subject LR11-328 (A) and LR11-147 (B) showing de novo status of mutations c.1073G>T and c.1088T>A in ATP1A3. The resulting amino acid substitutions Gly358Val (yellow spacefill) and Ile363Asn (blue spacefill) localize to the P domain of the ATP1A3 protein (red stick representation). A beta-strand (thin ribbon backbone highlighted white) connects the mutated residues to the active site phosphorylatable Asp366 (green spacefill). The structure is tilted to reveal the linear relationship between the mutations and the active site aspartate. The β subunit (green stick representation) is at the extracellular surface, and the yellow and gold A and N domains, like the red P domain, are cytoplasmic.