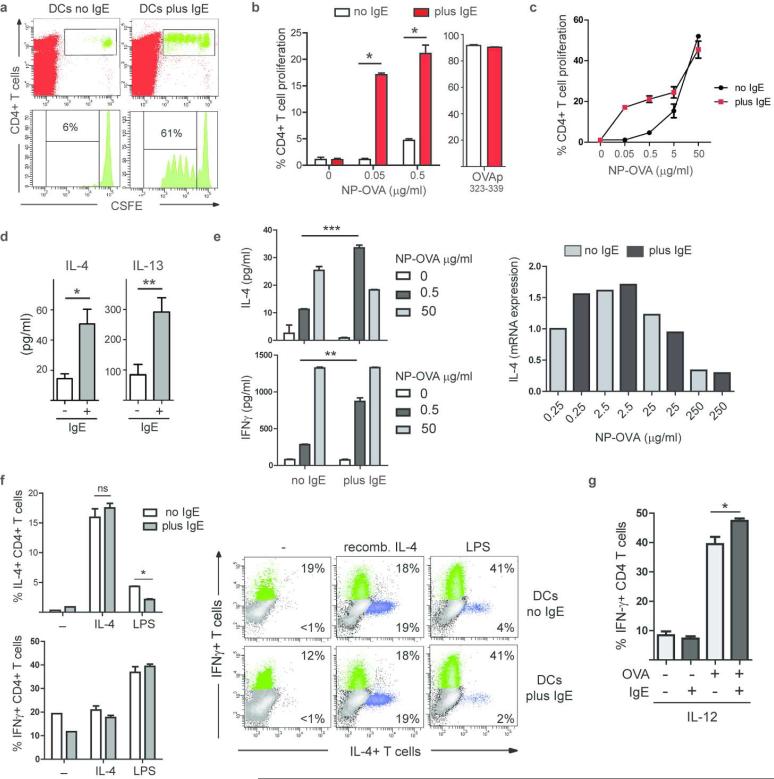
Figure 7.
IgE/FcεRI-mediated antigen presentation by DCs does not result in efficient generation of Th2-type effector T cells. (a) Induction of in vivo T cell proliferation. Splenic DCs were pulsed with NP-OVA in vitro (0.5 μg/ml antigen) in the presence (plus) or absence (no) of NP-specific IgE. Representative experiment (n=3). (b) Induction of in vitro T cell proliferation by DCs via IgE-mediated antigen uptake. Soluble OVA was present continuously; OVA-peptide323-339 was used as control. Mean of triplicates +/− SEM, representative experiment (n≥5). (c) In vitro DC/T cells proliferation assay in the presence of increasing antigen concentrations. Triplicates of representative experiment (n=2). (d) IL-4 and IL13 in DC/T cell co-culture supernatant at day 3. (e) IL-4 and IFN-γ increase in culture supernatants when DC-bound IgE is used for antigen uptake. Expression levels of IL-4 mRNA are compared in T cells that were induced IgE-independently (no IgE) or via the IgE/ FcεRI-mediated uptake (plus IgE). (f) Intracellular cytokine staining for IL-4 and IFN-γ in T cells. Recombinant IL-4 or LPS was added to the antigen presentation assays as indicated. Bar diagram shows percentages of IL-4+ and IFN-γ CD4+ T cells in DC/T cell co-cultures. Data represent triplicates of biological replicates +/− SEM of a representative experiment (n=3). Representative FACS blots depict gated CD4+ T cells at day 7. (g) Recombinant IL-12 was added to the co-cultures and priming of Th1 cells was determined by intracellular staining for IFN-γ at day 7. Data represent triplicates of biological replicates +/− SEM of a representative experiment (n=2).