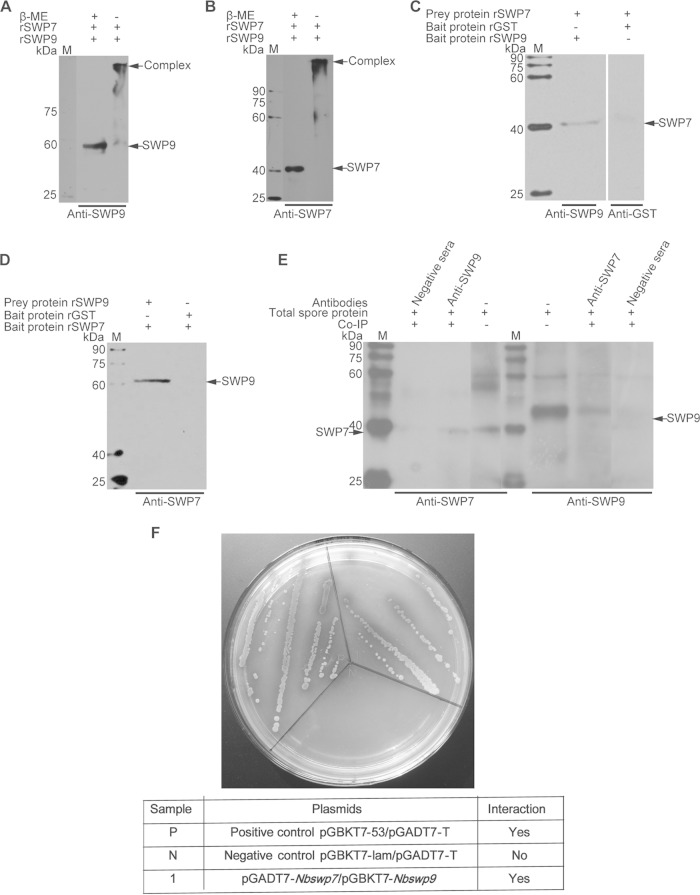
FIG 5.
Immunoprecipitation, far-Western blotting, and SDS-PAGE of unreduced, treated samples and yeast two-hybrid assay, showing the interaction of SWP9 and SWP7. (A and B) SDS-PAGE of the unreduced, treated sample for analysis of the interaction between SWP9 and SWP7. Shown are SDS-PAGE gels of protein samples made from rSWP9-GST and rSWP7-His treated with (+) or without (−) β-mercaptoethanol. Mixed protein samples of rSWP9-GST and rSWP7-His, when treated without β-mercaptoethanol, formed a large complex (arrow) at the top of the gel, as demonstrated by SDS-PAGE. (C and D) Detection of prey protein rSWP7-His or rSWP9-GST by conventional far-Western blotting using rSWP9-GST or rSWP7-His and anti-SWP9 or anti-SWP7 antibody. In addition, rGST and monoclonal anti-GST antibodies were used as controls. (E) Immunoblots of SWP7 and SWP9 were coimmunoprecipitated with rabbit anti-SWP9 and anti-SWP7 antibodies, respectively. The immunoprecipitates were treated with loading buffer and run on SDS-12% PAGE, transferred to a PVDF membrane, and probed with 10 μg mouse anti-SWP7 (1:6,000) or anti-SWP9 (1:6,000). The samples were incubated with HRP-labeled anti-mouse IgG (1:8,000) and detected by enhanced chemiluminescence (ECL). The negative-control antibody did not precipitate SWP9 and SWP7. Lanes M, EasySee Western marker. (F) A yeast two-hybrid assay was used to determine the in vivo interaction between SWP9 and SWP7. Nbswp7 (prey) and Nbswp9 (bait) constructs were simultaneously transformed into yeast competent cells. Several independent blue colonies grew up on the Leu− Trp− His− Ade− SD plates that contained X-α-Gal. This demonstrates the interactions of the pGADT7-Nbswp7 and pGBKT7-Nbswp9 constructs. The positive-control pGBKT7-53/pGADT7-T and negative-control pGBKT7-lam/pGADT7-T reactions are also shown.