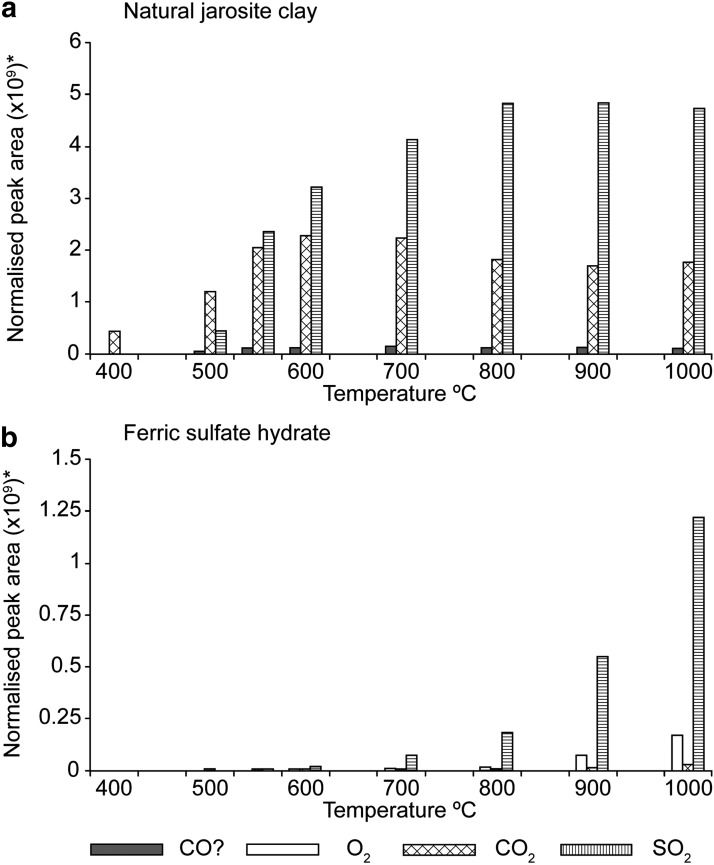
FIG. 4.
The production of sulfur dioxide, carbon dioxide, possible carbon monoxide, and oxygen during the thermal decomposition of samples of natural jarosite clay and a lab standard of ferric sulfate hydrate in individual heating experiments carried out between 400°C and 1000°C at 100°C increments. *The peak areas were normalized by sulfate mass (100% in ferric sulfate hydrate, 5% of sample mass in the natural jarosite clay—from XRD results). The m/z 28 peak is labeled as CO?, as it could be either carbon monoxide or nitrogen; we infer carbon monoxide as discussed in the text.